Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory
- PMID: 21734285
- PMCID: PMC3758574
- DOI: 10.1523/JNEUROSCI.1062-11.2011
Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory
Abstract
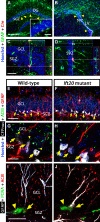
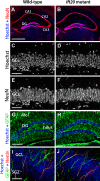
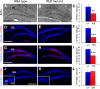
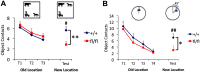
Integration of new neurons into the adult hippocampus has been linked to specific types of learning. Primary cilia were found to be required for the formation of adult neural stem cells (NSCs) in the hippocampal dentate gyrus during development. However, the requirement of cilia in maintenance of adult NSCs is unknown. We developed a genetic mouse model in which fetal/perinatal brain development is unaffected, but adult hippocampal neurogenesis is constantly reduced by conditional ablation of primary cilia in adult GFAP(+) neural stem/progenitor cells. We found that this approach specifically reduces the number of hippocampal amplifying progenitors (also called type 2a cells) without affecting the number of radial NSCs (or type 1 cells). Constant reduction of adult hippocampal neurogenesis produced a delay rather than a permanent deficiency in spatial learning without affecting the retention of long-term memories. Decreased neurogenesis also altered spatial novelty recognition and hippocampus-independent cue conditioning. Here, we propose that adult hippocampal newborn neurons increase the efficiency of generating the new representations of spatial memories and that reduction of adult hippocampal neurogenesis may be biased toward cue-based strategies. This novel mouse model provides evidences that cognitive deficits associated with ciliary defects (ciliopathies) might be, in part, mediated by the deficiency of primary cilia in adult hippocampal stem/progenitor cells.
Figures




References
-
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. - PubMed
-
- Bach ME, Hawkins RD, Osman M, Kandel ER, Mayford M. Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the theta frequency. Cell. 1995;81:905–915. - PubMed
-
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18:64–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
