Hepatic late adverse effects after antineoplastic treatment for childhood cancer
- PMID: 21735424
- PMCID: PMC6464972
- DOI: 10.1002/14651858.CD008205.pub2
Hepatic late adverse effects after antineoplastic treatment for childhood cancer
Update in
-
Hepatic late adverse effects after antineoplastic treatment for childhood cancer.Cochrane Database Syst Rev. 2019 Apr 15;4(4):CD008205. doi: 10.1002/14651858.CD008205.pub3. Cochrane Database Syst Rev. 2019. PMID: 30985922 Free PMC article.
Abstract
Background: Survival rates have greatly improved as a result of more effective treatments for childhood cancer. Unfortunately the improved prognosis has resulted in the occurrence of late, treatment-related complications. Liver complications are common during and soon after treatment for childhood cancer. However, among long-term childhood cancer survivors the risk of hepatic late adverse effects is largely unknown. To make informed decisions about future cancer treatment and follow-up policies it is important to know the risk of, and associated risk factors for, hepatic late adverse effects.
Objectives: To evaluate the existing evidence on the association between antineoplastic treatment for childhood cancer and hepatic late adverse effects.
Search strategy: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 2), MEDLINE (1966 to June 2009) and EMBASE (1980 to June 2009). In addition, we searched reference lists of relevant articles and conference proceedings.
Selection criteria: All studies except case reports, case series and studies including less than 10 patients that examined the association between antineoplastic treatment for childhood cancer (aged 18 years or less at diagnosis) and hepatic late adverse effects (one year or more after the end of treatment).
Data collection and analysis: Two review authors independently performed the study selection, risk of bias assessment and data extraction.
Main results: We identified 20 cohort studies investigating hepatic late adverse effects after antineoplastic treatment for childhood cancer. All studies had methodological limitations. The prevalence of hepatic late adverse effects varied widely, between 0% and 84.2%. Selecting studies where the outcome of hepatic late adverse effects was well defined as alanine aminotransferase (ALT) above the upper limit of normal resulted in five studies. In this subgroup the prevalence of hepatic late adverse effects ranged from 8.0% to 52.8%, with follow-up durations varying from one to 27 years after the end of treatment. A more stringent selection process using the outcome definition of ALT as above twice the upper limit of normal resulted in three studies, with a prevalence ranging from 7.9% to 44.8%. Chronic viral hepatitis was identified as a risk factor for hepatic late adverse effects in univariate analyses. It is unclear which specific antineoplastic treatments increase the risk of hepatic late adverse effects
Authors' conclusions: The prevalence of hepatic late adverse effects ranged from 7.9% to 52.8% when selecting studies with an adequate outcome definition. It has not been established which childhood cancer treatments result in hepatic late adverse effects. There is a suggestion that chronic viral hepatitis increases the risk of hepatic late adverse effects. More well-designed studies are needed to reliably evaluate the prevalence of, and risk factors for, hepatic late adverse effects after antineoplastic treatment for childhood cancer.
Conflict of interest statement
Dorine Bresters is an author of a study included in this systematic review.
Figures


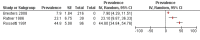


References
References to studies included in this review
-
- Aricò M, Maggiore G, Silini E, Bono F, Vigano C, Cerino A, et al. Hepatitis C virus infection in children treated for acute lymphoblastic leukemia. Blood 1994;84:2919‐22. - PubMed
-
- Ballauff A, Krahe J, Jansen B, Ross RS, Roggendorf H, Havers W. Chronic liver disease after treatment of malignancies in children [Chronische Hepatopathien nach Behandlung maligner Erkrankungen bei Kindern]. Klinische Pädiatrie 1999;211:49‐52. - PubMed
-
- Bessho F, Kinumaki H, Yokota S, Hayashi Y, Kobayashi M, Kamoshita S. Liver function studies in children with acute lymphocytic leukemia after cessation of therapy. Medical and Pediatric Oncology 1994;23:111‐5. - PubMed
-
- Bresters D, Gils IC, Dekker FW, Lankester AC, Bredius RG, Schweizer JJ. Abnormal liver enzymes two years after haematopoietic stem cell transplantation in children: prevalence and risk factors. Bone Marrow Transplantation 2008;41:27‐31. - PubMed
-
- Chotsampancharoen T, Gan K, Kasow KA, Barfield RC, Hale GA, Leung W. Iron overload in survivors of childhood leukemia after allogeneic hematopoietic stem cell transplantation. Pediatric Transplantation 2009;13:348‐52. - PubMed
References to studies excluded from this review
-
- Adson MA, Weiland LH. Resection of primary solid hepatic tumors. American Journal of Surgery 1981;141:18‐21. - PubMed
-
- Amylon MD, Co JPT, Snyder DS, Donaldson SS, Blume KG, Forman SJ. Allogeneic bone marrow transplant in pediatric patients with high‐risk hematopoietic malignancies early in the course of their disease. Journal of Pediatric Hematology/Oncology 1997;19:54‐61. - PubMed
-
- Atay AA, Kurekci AE, Kesik V, Kilic S, Gulgun M, Ozcan O, et al. Retrospective analysis of children with acute lymphoblastic leukemia [Akut lenfoblastik losemili olgularimizin retrospektif analizi]. Gulhane Medical Journal 2005;47:183‐6.
-
- Avet Loiseau H, Hartmann O, Valteau D, McDowell H, Brugieres L, Vassal G, et al. High‐dose chemotherapy containing busulfan followed by bone marrow transplantation in 24 children with refractory or relapsed non‐Hodgkin's lymphoma. Bone Marrow Transplantation 1991;8:465‐72. - PubMed
References to studies awaiting assessment
-
- Kovacs G, Sapi C, Csoka M, Erdelyi D, Haltrich I, Muller J. Hepatotoxic late side effects in children with haematological malignancies. Pediatric Blood and Cancer 2007;49:547‐8.
-
- Kristinsson VH, Kristinsson JR, Jonmundsson GK, Jonsson OG, Porsson AV, Haraldsson A. Late and long‐term effects of leukemia treatment in childhood [Siokmnar of langvinnar aukaverkanir eftir hvitblaeoismeofero i aesku]. Laeknabladid 2002;88:13‐8. - PubMed
-
- Thavaraj V, Seth R, Arya LS, Kumar R. Screening for problems of pediatric cancer survivors in our center. Pediatic Blood and Cancer 2006;47:481.
Additional references
-
- Baldo V, Baldovin T, Trivello R, Floreani A. Epidemiology of HCV infection. Current Pharmaceutical Design 2008;14:1646‐54. - PubMed
-
- Castellino S, Lensing S, Riely C, Rai SN, Davila R, Hayden RT, et al. The epidemiology of chronic hepatitis C infection in survivors of childhood cancer: an update of the St Jude Children's Research Hospital hepatitis C seropositive cohort. Blood 2004;103:2460‐6. - PubMed
-
- Curry HL, Parkes SE, Powell JE, Mann JR. Caring for survivors of childhood cancer: the size of the problem. European Journal of Cancer 2006;42:501‐8. - PubMed
-
- Dawson LA, Haken RK. Partial volume tolerance of the liver to radiation. Seminars in Radiation Oncology 2005;15:279‐83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

