Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo
- PMID: 21735478
- PMCID: PMC3192242
- DOI: 10.1002/jbmr.465
Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo
Abstract
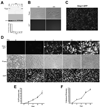
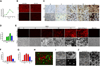
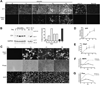
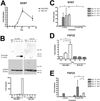
Osteocytes are the most abundant cells in bone yet are the most challenging to study because they are embedded in a mineralized matrix. We generated a clonal cell line called IDG-SW3 (for Immortomouse/Dmp1-GFP-SW3) from long-bone chips from mice carrying a Dmp1 promoter driving GFP crossed with the Immortomouse, which expresses a thermolabile SV40 large T antigen regulated by interferon γ (IFN-γ). Cells from these mice can be expanded at 33 °C in the presence of IFN-γ and then allowed to resume their original phenotype at 37 °C in the absence of IFN-γ. IDG-SW3 cells are Dmp1-GFP(-) and T antigen(+) under immortalizing conditions but Dmp1-GFP(+) and T antigen(-) under osteogenic conditions. Like osteoblasts, they express alkaline phosphatase and produce and mineralize a type 1 collagen matrix containing calcospherulites. Like early osteocytes, they express E11/gp38, Dmp1, MEPE, and Phex. Like late osteocytes, they develop a dendritic morphology and express SOST/sclerostin and fibroblast growth factor 23 (FGF-23), regulated by parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D(3). When cultured on 3D matrices, they express Dmp1-GFP and sclerostin. When the 3D cultures are implanted in calvarial defects in vivo, they accelerate bone healing. This cell line should prove useful for studying osteoblast-to-osteocyte transition, mechanisms for biomineralization, osteocyte function, and regulation of SOST/sclerostin and FGF-23.
Copyright © 2011 American Society for Bone and Mineral Research.
Conflict of interest statement
CONFLICT OF INTEREST
All authors have no conflicts of interest.
Figures


References
-
- Robling AG, Bellido T, Turner CH. Mechanical stimulation in vivo reduces osteocyte expression of sclerostin. J Musculoskelet Neuronal Interact. 2006;6(4):354. - PubMed
-
- Gluhak-Heinrich J, Ye L, Bonewald LF, Feng JQ, MacDougall M, Harris SE, Pavlin D. Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo. J Bone Miner Res. 2003;18(5):807–817. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

