Evaluation of long-term antinociceptive properties of stabilized hyaluronic acid preparation (NASHA) in an animal model of repetitive joint pain
- PMID: 21736716
- PMCID: PMC3239347
- DOI: 10.1186/ar3394
Evaluation of long-term antinociceptive properties of stabilized hyaluronic acid preparation (NASHA) in an animal model of repetitive joint pain
Abstract
Introduction: Clinical trials provided controversial results on whether the injection of hyaluronan preparations into osteoarthritic joints reduces pain. Problems of clinical studies may be the substantial placebo effects of intra-articular injections, different severity and rate of progression of the disease and others. We hypothesize that the use of preclinical pain models may help to clarify whether a certain hyaluronan exerts antinociceptive effects upon intra-articular injection. In the present study we tested in the bradykinin/prostaglandin E(2) (PGE(2)) model primarily the putative antinociceptive effect of stabilized hyaluronic acid from a non animal source (NASHA), a stabilized hyaluronic acid based gel for intra-articular treatment of OA. We established a dose-response relationship for NASHA and we compared NASHA to other hyaluronans with different formulations that are in clinical use.
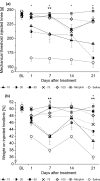
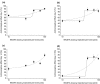
Methods: To induce transient joint pain episodes bradykinin and PGE(2) were repetitively administered intra-articularly and unilaterally into rat knee joints during short anaesthesia. After establishment of the predrug nociceptive responses, a single intra-articular injection of saline or NASHA at different concentrations was administered and pain responses to further bradykinin/PGE(2) injections were monitored up to 56 days after NASHA. Furthermore, the obtained effective dose was compared to clinically defined concentrations of Hylan GF20 and sodium hyaluronate. The primary outcome measures were primary mechanical hyperalgesia at the knee joint and pain-induced weight bearing.
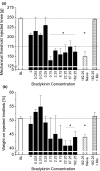
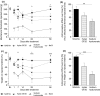
Results: On day 1 after injection, all tested hyaluronan preparations showed an antinociceptive effect >50% compared to saline. Single injections of higher doses of NASHA (50, 75 and 100 μl) were antinociceptive up to 56 days. When injection volumes in rat knee joints were adapted to clinical injection volumes in humans, the antinociceptive effects of the cross-linked NASHA and Hylan GF20 had a longer duration than that of the non cross-linked sodium hyaluronate (with a slightly better effect of NASHA than Hylan GF20).
Conclusions: In the bradykinin/PGE(2) model of joint pain a single injection of all hyaluronan preparations provided significant antinociceptive effects compared to saline. It appeared that the duration of the antinociceptive effect of the cross-linked hyaluronan preparations NASHA and Hylan GF20 was more prolonged. In addition, the gel beads structure allowing only a slow release of hyaluronic acid (NASHA) may even enhance this prolonged antinociceptive effect.
Figures
References
-
- Steinmeyer J. Drug therapy of arthrosis. Orthopäde. 2001;30:856–865. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

