Protocol for the Cognitive Interventions and Nutritional Supplements (CINS) trial: a randomized controlled multicenter trial of a brief intervention (BI) versus a BI plus cognitive behavioral treatment (CBT) versus nutritional supplements for patients with long-lasting muscle and back pain
- PMID: 21736730
- PMCID: PMC3146910
- DOI: 10.1186/1471-2474-12-152
Protocol for the Cognitive Interventions and Nutritional Supplements (CINS) trial: a randomized controlled multicenter trial of a brief intervention (BI) versus a BI plus cognitive behavioral treatment (CBT) versus nutritional supplements for patients with long-lasting muscle and back pain
Abstract
Background: Brief intervention programs are clinically beneficial, and cost efficient treatments for low back pain, when offered at 8-12 weeks, compared with treatment as usual. However, about 30% of the patients do not return to work. The European Guidelines for treatment of chronic low back pain recommends Cognitive Behavioral Therapy (CBT), but conclude that further research is needed to evaluate the effectiveness of CBT for chronic low back pain.
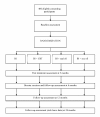
Methods/design: The aim of the multicenter CINS trial (Cognitive Interventions and Nutritional Supplements) is to compare the effectiveness of 4 different interventions; Brief Intervention, Brief Intervention and CBT, Brief Intervention and nutritional supplements of seal oil, and Brief Intervention and nutritional supplements of soy oil. All participants will be randomly assigned to the interventions. The nutritional supplements will be tested in a double blind design. 400 patients will be recruited from a population of chronic low back pain patients that have been sick listed for 2-10 months. Four outpatient clinics, located in different parts of Norway, will participate in recruitment and treatment of the patients.The Brief Intervention is a one session cognitive, clinical examination program based on a non-injury model, where return to normal activity and work is the main goal, and is followed by two booster sessions. The CBT is a tailored treatment involving 7 sessions, following a detailed manual. The nutritional supplements consist of a dosage of 10 grams of either soy or seal oil (capsules) per day for 3 months, administered in a double blind design. All patients will be followed up with questionnaires after 3, 6 and 12 months, while sick leave data will be collected up to at least 24 months after randomization. The primary outcome of the study is sick leave and will be based on register data from the National Insurance Administration. Secondary outcomes include self-reported data on disability, pain, and psychological variables.
Conclusions: To our knowledge, the CINS trial will be the largest, randomized trial of psychological and nutritional interventions for chronic low back pain patients to date. It will provide important information regarding the effectiveness of CBT and seal oil for chronic low back pain patients.
Trial registration: http://www.clinicaltrials.gov, with registration number NCT00463970.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical