Efficacy and safety of the human anti-IL-1β monoclonal antibody canakinumab in rheumatoid arthritis: results of a 12-week, Phase II, dose-finding study
- PMID: 21736751
- PMCID: PMC3152943
- DOI: 10.1186/1471-2474-12-153
Efficacy and safety of the human anti-IL-1β monoclonal antibody canakinumab in rheumatoid arthritis: results of a 12-week, Phase II, dose-finding study
Abstract
Background: Canakinumab is a fully human anti-interleukin IL-1beta monoclonal antibody, being investigated for the treatment of rheumatoid arthritis (RA). This multicenter, phase II, randomized, double-blind, placebo-controlled, parallel-group, dose-finding study investigated the efficacy and safety of canakinumab in patients with active RA despite ongoing therapy at stable doses of methotrexate.
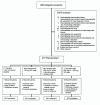
Methods: Patients were randomized to receive one of four regimens, in addition to methotrexate, for 12 weeks: canakinumab 150 mg subcutaneously (SC) every 4 weeks (q4wk), canakinumab 300 mg SC (2 injections of 150 mg SC) every 2 weeks, a 600 mg intravenous loading dose of canakinumab followed by 300 mg SC every 2 weeks', or placebo SC every 2 weeks.
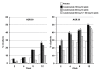
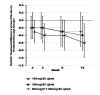
Results: Among 274 patients with evaluable efficacy data, the percentage of responders according to American College of Rheumatology 50 criteria (the primary endpoint, based on a 28-joint count) was significantly higher with canakinumab 150 mg SC q4wk than with placebo (26.5% vs. 11.4%, respectively; p = 0.028). Compared to placebo, this dosage of canakinumab was also associated with significantly more favorable responses at week 12 with respect to secondary endpoints including the Disease Activity Score 28, scores on the Health Assessment Questionnaire and Functional Assessment of Chronic Illness Therapy-Fatigue, swollen 28-joint count, and patient's and physician's global assessments of disease activity. No safety concerns were raised with canakinumab therapy, particularly with regard to infections. Few injection-site reactions occurred.
Conclusion: The addition of canakinumab 150 mg SC q4wk improves therapeutic responses among patients who have active RA despite stable treatment with methotrexate.
Trial registration: (ClinicalTrials.gov identifier: NCT00784628).
Figures
References
-
- Dayer JM. The pivotal role of interleukin-1 in the clinical manifestations of rheumatoid arthritis. Rheumatology (Oxford) 2003;42(suppl 2):ii3–10. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

