cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms
- PMID: 21737330
- PMCID: PMC3163271
- DOI: 10.1016/j.molcel.2011.06.011
cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms
Abstract
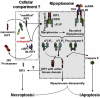
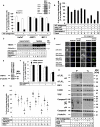
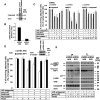
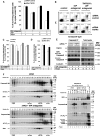
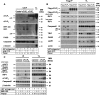
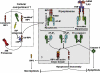
The intracellular regulation of cell death pathways by cIAPs has been enigmatic. Here we show that loss of cIAPs promotes the spontaneous formation of an intracellular platform that activates either apoptosis or necroptosis. This 2 MDa intracellular complex that we designate "Ripoptosome" is necessary but not sufficient for cell death. It contains RIP1, FADD, caspase-8, caspase-10, and caspase inhibitor cFLIP isoforms. cFLIP(L) prevents Ripoptosome formation, whereas, intriguingly, cFLIP(S) promotes Ripoptosome assembly. When cIAPs are absent, caspase activity is the "rheostat" that is controlled by cFLIP isoforms in the Ripoptosome and decides if cell death occurs by RIP3-dependent necroptosis or caspase-dependent apoptosis. RIP1 is the core component of the complex. As exemplified by our studies for TLR3 activation, our data argue that the Ripoptosome critically influences the outcome of membrane-bound receptor triggering. The differential quality of cell death mediated by the Ripoptosome may cause important pathophysiological consequences during inflammatory responses.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


Comment in
-
The Ripoptosome: death decision in the cytosol.Mol Cell. 2011 Aug 5;43(3):323-5. doi: 10.1016/j.molcel.2011.07.007. Mol Cell. 2011. PMID: 21816342
-
A new platform for death.Nat Rev Mol Cell Biol. 2011 Aug 18;12(9):547. doi: 10.1038/nrm3174. Nat Rev Mol Cell Biol. 2011. PMID: 21850033 No abstract available.
References
-
- Bertrand M.J., Milutinovic S., Dickson K.M., Ho W.C., Boudreault A., Durkin J., Gillard J.W., Jaquith J.B., Morris S.J., Barker P.A. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell. 2008;30:689–700. - PubMed
-
- Biton S., Ashkenazi A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-alpha feedforward signaling. Cell. 2011;145:92–103. - PubMed
-
- Budd R.C., Yeh W.C., Tschopp J. cFLIP regulation of lymphocyte activation and development. Nat. Rev. Immunol. 2006;6:196–204. - PubMed
-
- Cain K., Brown D.G., Langlais C., Cohen G.M. Caspase activation involves the formation of the aposome, a large (approximately 700 kDa) caspase-activating complex. J. Biol. Chem. 1999;274:22686–22692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

