Activation of the bumetanide-sensitive Na+,K+,2Cl- cotransporter (NKCC2) is facilitated by Tamm-Horsfall protein in a chloride-sensitive manner
- PMID: 21737451
- PMCID: PMC3191059
- DOI: 10.1074/jbc.M111.222968
Activation of the bumetanide-sensitive Na+,K+,2Cl- cotransporter (NKCC2) is facilitated by Tamm-Horsfall protein in a chloride-sensitive manner
Abstract
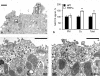
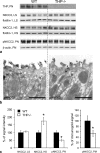
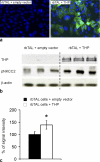
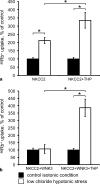
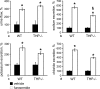
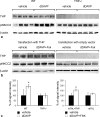
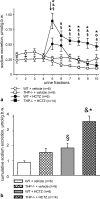
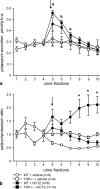
Active transport of NaCl across thick ascending limb (TAL) epithelium is accomplished by Na(+),K(+),2Cl(-) cotransporter (NKCC2). The activity of NKCC2 is determined by vasopressin (AVP) or intracellular chloride concentration and includes its amino-terminal phosphorylation. Co-expressed Tamm-Horsfall protein (THP) has been proposed to interact with NKCC2. We hypothesized that THP modulates NKCC2 activity in TAL. THP-deficient mice (THP(-/-)) showed an increased abundance of intracellular NKCC2 located in subapical vesicles (+47% compared with wild type (WT) mice), whereas base-line phosphorylation of NKCC2 was significantly decreased (-49% compared with WT mice), suggesting reduced activity of the transporter in the absence of THP. Cultured TAL cells with low endogenous THP levels and low base-line phosphorylation of NKCC2 displayed sharp increases in NKCC2 phosphorylation (+38%) along with a significant change of intracellular chloride concentration upon transfection with THP. In NKCC2-expressing frog oocytes, co-injection with THP cRNA significantly enhanced the activation of NKCC2 under low chloride hypotonic stress (+112% versus +235%). Short term (30 min) stimulation of the vasopressin V2 receptor pathway by V2 receptor agonist (deamino-cis-D-Arg vasopressin) resulted in enhanced NKCC2 phosphorylation in WT mice and cultured TAL cells transfected with THP, whereas in the absence of THP, NKCC2 phosphorylation upon deamino-cis-D-Arg vasopressin was blunted in both systems. Attenuated effects of furosemide along with functional and structural adaptation of the distal convoluted tubule in THP(-/-) mice supported the notion that NaCl reabsorption was impaired in TAL lacking THP. In summary, these results are compatible with a permissive role for THP in the modulation of NKCC2-dependent TAL salt reabsorptive function.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

