Activation and regulation of purinergic P2X receptor channels
- PMID: 21737531
- PMCID: PMC3141880
- DOI: 10.1124/pr.110.003129
Activation and regulation of purinergic P2X receptor channels
Abstract
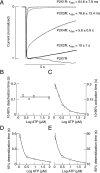
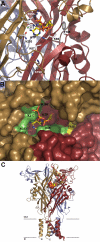
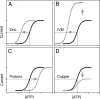
Mammalian ATP-gated nonselective cation channels (P2XRs) can be composed of seven possible subunits, denoted P2X1 to P2X7. Each subunit contains a large ectodomain, two transmembrane domains, and intracellular N and C termini. Functional P2XRs are organized as homomeric and heteromeric trimers. This review focuses on the binding sites involved in the activation (orthosteric) and regulation (allosteric) of P2XRs. The ectodomains contain three ATP binding sites, presumably located between neighboring subunits and formed by highly conserved residues. The detection and coordination of three ATP phosphate residues by positively charged amino acids are likely to play a dominant role in determining agonist potency, whereas an AsnPheArg motif may contribute to binding by coordinating the adenine ring. Nonconserved ectodomain histidines provide the binding sites for trace metals, divalent cations, and protons. The transmembrane domains account not only for the formation of the channel pore but also for the binding of ivermectin (a specific P2X4R allosteric regulator) and alcohols. The N- and C- domains provide the structures that determine the kinetics of receptor desensitization and/or pore dilation and are critical for the regulation of receptor functions by intracellular messengers, kinases, reactive oxygen species and mercury. The recent publication of the crystal structure of the zebrafish P2X4.1R in a closed state provides a major advance in the understanding of this family of receptor channels. We will discuss data obtained from numerous site-directed mutagenesis experiments accumulated during the last 15 years with reference to the crystal structure, allowing a structural interpretation of the molecular basis of orthosteric and allosteric ligand actions.
Figures




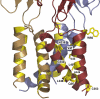
References
-
- Acuña-Castillo C, Coddou C, Bull P, Brito J, Huidobro-Toro JP. (2007) Differential role of extracellular histidines in copper, zinc, magnesium and proton modulation of the P2X7 purinergic receptor. J Neurochem 101:17–26 - PubMed
-
- Acuña-Castillo C, Morales B, Huidobro-Toro JP. (2000) Zinc and copper modulate differentially the P2X4 receptor. J Neurochem 74:1529–1537 - PubMed
-
- Adriouch S, Bannas P, Schwarz N, Fliegert R, Guse AH, Seman M, Haag F, Koch-Nolte F. (2008) ADP-ribosylation at R125 gates the P2X7 ion channel by presenting a covalent ligand to its nucleotide binding site. FASEB J 22:861–869 - PubMed
-
- Agboh KC, Webb TE, Evans RJ, Ennion SJ. (2004) Functional characterization of a P2X receptor from Schistosoma mansoni. J Biol Chem 279:41650–41657 - PubMed
-
- Ahrens J, Leuwer M, Demir R, Krampfl K, Foadi N, Haeseler G. (2008) The anaesthetic steroid alphaxalone positively modulates alpha1-glycine receptor function. Pharmacology 82:228–232 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

