A new VCAN/versican splice acceptor site mutation in a French Wagner family associated with vascular and inflammatory ocular features
- PMID: 21738396
- PMCID: PMC3130719
A new VCAN/versican splice acceptor site mutation in a French Wagner family associated with vascular and inflammatory ocular features
Abstract
Purpose: To detail the highly variable ocular phenotypes of a French family affected with an autosomal dominantly inherited vitreoretinopathy and to identify the disease gene.
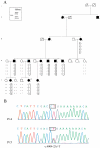
Methods: Sixteen family members with ten affected individuals underwent detailed ophthalmic evaluation. Genetic linkage analysis and gene screening were undertaken for genes known to be involved in degenerative and exudative vitreoretinopathies. Qualitative reverse transcriptase-PCR analysis of the versiscan (VCAN) transcripts was performed after mutation detection in the VCAN gene.
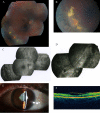
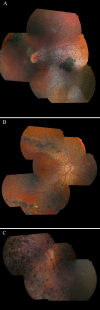
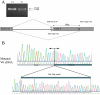
Results: The first index patient of this French family was referred to us because of a chronic uveitis since infancy; this uveitis was associated with exudative retinal detachment in the context of a severe uncharacterized familial vitreoretinopathy. Genetic linkage was obtained to the VCAN locus, and we further identified a new pathogenic mutation at the highly conserved splice acceptor site in intron 7 of the VCAN gene (c.4004-2A>T), which produced aberrantly spliced VCAN transcripts.
Conclusions: Extensive molecular investigation allowed us to classify this familial vitreoretinopathy as Wagner syndrome. This study illustrates the need to confirm clinical diagnosis by molecular genetic testing and adds new ocular phenotypes to the Wagner syndrome, such as vascular and inflammatory features.
Figures
References
-
- Graemiger RA, Niemeyer G, Schneeberger SA, Messmer EP. Wagner vitreoretinal degeneration. Follow-up of the original pedigree. Ophthalmology. 1995;102:1830–9. - PubMed
-
- Wagner H. Ein bisher unbekanntes Erbleiden des Auges (degeneratiohyaloideo-retinalis hereditaria), beobachtet im Kanton Zurich. Klin Mbl Augenheilk. 1938;100:840–57.
-
- Stickler GB, Belau PG, Farrell FJ, Jones JD, Pugh DG, Steinberg AG, Ward LE. Hereditary progressive arthro-ophthalmopathy. Mayo Clin Proc. 1965;40:433–55. - PubMed
-
- Black GC, Perveen R, Wiszniewski W, Dodd CL, Donnai D, McLeod D. A novel hereditary developmental vitreoretinopathy with multiple ocular abnormalities localizing to a 5-cM region of chromosome 5q13-q14. Ophthalmology. 1999;106:2074–81. - PubMed
-
- Brown DM, Graemiger RA, Hergersberg M, Schinzel A, Messmer EP, Niemeyer G, Schneeberger SA, Streb LM, Taylor CM, Kimura AE. Genetic linkage of Wagner disease and erosive vitreoretinopathy to chromosome 5q13–14. Arch Ophthalmol. 1995;113:671–5. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
