The binding of triclosan to SmeT, the repressor of the multidrug efflux pump SmeDEF, induces antibiotic resistance in Stenotrophomonas maltophilia
- PMID: 21738470
- PMCID: PMC3128119
- DOI: 10.1371/journal.ppat.1002103
The binding of triclosan to SmeT, the repressor of the multidrug efflux pump SmeDEF, induces antibiotic resistance in Stenotrophomonas maltophilia
Abstract
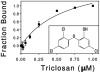
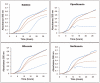
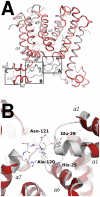
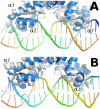
The wide utilization of biocides poses a concern on the impact of these compounds on natural bacterial populations. Furthermore, it has been demonstrated that biocides can select, at least in laboratory experiments, antibiotic resistant bacteria. This situation has raised concerns, not just on scientists and clinicians, but also on regulatory agencies, which are demanding studies on the impact that the utilization of biocides may have on the development on resistance and consequently on the treatment of infectious diseases and on human health. In the present article, we explored the possibility that the widely used biocide triclosan might induce antibiotic resistance using as a model the opportunistic pathogen Stenotrophomonas maltophilia. Biochemical, functional and structural studies were performed, focusing on SmeDEF, the most relevant antibiotic- and triclosan-removing multidrug efflux pump of S. maltophilia. Expression of smeDEF is regulated by the repressor SmeT. Triclosan released SmeT from its operator and induces the expression of smeDEF, thus reducing the susceptibility of S. maltophilia to antibiotics in the presence of the biocide. The structure of SmeT bound to triclosan is described. Two molecules of triclosan were found to bind to one subunit of the SmeT homodimer. The binding of the biocide stabilizes the N terminal domain of both subunits in a conformation unable to bind DNA. To our knowledge this is the first crystal structure obtained for a transcriptional regulator bound to triclosan. This work provides the molecular basis for understanding the mechanisms allowing the induction of phenotypic resistance to antibiotics by triclosan.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


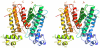


References
-
- Aiello AE, Larson EL, Levy SB. Consumer antibacterial soaps: effective or just risky? Clin Infect Dis. 2007;45(Suppl 2):S137–147. - PubMed
-
- De Muynck W, De Belie N, Verstraete W. Antimicrobial mortar surfaces for the improvement of hygienic conditions. J Appl Microbiol. 2010;108:62–72. - PubMed
-
- Moretro T, Sonerud T, Mangelrod E, Langsrud S. Evaluation of the antibacterial effect of a triclosan-containing floor used in the food industry. J Food Prot. 2006;69:627–633. - PubMed
-
- Jones RD, Jampani HB, Newman JL, Lee AS. Triclosan: a review of effectiveness and safety in health care settings. Am J Infect Control. 2000;28:184–196. - PubMed
-
- Sjoblom M, Ainamo A, Ainamo J. Antimicrobial effect of four different toothpastes. Scand J Dent Res. 1976;84:377–380. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical

