A dynamic landscape for antibody binding modulates antibody-mediated neutralization of West Nile virus
- PMID: 21738473
- PMCID: PMC3128118
- DOI: 10.1371/journal.ppat.1002111
A dynamic landscape for antibody binding modulates antibody-mediated neutralization of West Nile virus
Abstract
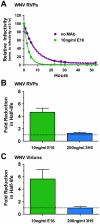
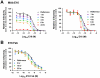
Neutralizing antibodies are a significant component of the host's protective response against flavivirus infection. Neutralization of flaviviruses occurs when individual virions are engaged by antibodies with a stoichiometry that exceeds a required threshold. From this "multiple-hit" perspective, the neutralizing activity of antibodies is governed by the affinity with which it binds its epitope and the number of times this determinant is displayed on the surface of the virion. In this study, we investigated time-dependent changes in the fate of West Nile virus (WNV) decorated with antibody in solution. Experiments with the well-characterized neutralizing monoclonal antibody (MAb) E16 revealed a significant increase in neutralization activity over time that could not be explained by the kinetics of antibody binding, virion aggregation, or the action of complement. Additional kinetic experiments using the fusion-loop specific MAb E53, which has limited neutralizing activity because it recognizes a relatively inaccessible epitope on mature virions, identified a role of virus "breathing" in regulating neutralization activity. Remarkably, MAb E53 neutralized mature WNV in a time- and temperature-dependent manner. This phenomenon was confirmed in studies with a large panel of MAbs specific for epitopes in each domain of the WNV envelope protein, with sera from recipients of a live attenuated WNV vaccine, and in experiments with dengue virus. Given enough time, significant inhibition of infection was observed even for antibodies with very limited, or no neutralizing activity in standard neutralization assays. Together, our data suggests that the structural dynamics of flaviviruses impacts antibody-mediated neutralization via exposure of otherwise inaccessible epitopes, allowing for antibodies to dock on the virion with a stoichiometry sufficient for neutralization.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Lindenbach BD, Thiel HJ, Rice CM. Flaviviridae: The Viruses and Their Replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Philadelphia: Lippincott-Williams & Wilkins; 2007. pp. 1101–1152.
-
- Sejvar JJ. The long-term outcomes of human West Nile virus infection. Clin Infect Dis. 2007;44:1617–1624. - PubMed
-
- Fernandez-Garcia MD, Mazzon M, Jacobs M, Amara A. Pathogenesis of flavivirus infections: using and abusing the host cell. Cell Host Microbe. 2009;5:318–328. - PubMed
-
- Brinton MA. The molecular biology of West Nile Virus: a new invader of the western hemisphere. Annu Rev Microbiol. 2002;56:371–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

