Identification of nine novel loci associated with white blood cell subtypes in a Japanese population
- PMID: 21738478
- PMCID: PMC3128095
- DOI: 10.1371/journal.pgen.1002067
Identification of nine novel loci associated with white blood cell subtypes in a Japanese population
Abstract
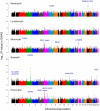
White blood cells (WBCs) mediate immune systems and consist of various subtypes with distinct roles. Elucidation of the mechanism that regulates the counts of the WBC subtypes would provide useful insights into both the etiology of the immune system and disease pathogenesis. In this study, we report results of genome-wide association studies (GWAS) and a replication study for the counts of the 5 main WBC subtypes (neutrophils, lymphocytes, monocytes, basophils, and eosinophils) using 14,792 Japanese subjects enrolled in the BioBank Japan Project. We identified 12 significantly associated loci that satisfied the genome-wide significance threshold of P<5.0×10(-8), of which 9 loci were novel (the CDK6 locus for the neutrophil count; the ITGA4, MLZE, STXBP6 loci, and the MHC region for the monocyte count; the SLC45A3-NUCKS1, GATA2, NAALAD2, ERG loci for the basophil count). We further evaluated associations in the identified loci using 15,600 subjects from Caucasian populations. These WBC subtype-related loci demonstrated a variety of patterns of pleiotropic associations within the WBC subtypes, or with total WBC count, platelet count, or red blood cell-related traits (n = 30,454), which suggests unique and common functional roles of these loci in the processes of hematopoiesis. This study should contribute to the understanding of the genetic backgrounds of the WBC subtypes and hematological traits.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Ronald H, Edward JB, Sanford JS, Bruce F, Leslie E, et al. Hematology: basic principles and practice (5th edition) Churchill: Livingstone/Elsevie. 2009.
-
- Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc. 2009;6:256–259. - PubMed
-
- Arinobu Y, Iwasaki H, Akashi K. Origin of basophils and mast cells. Allergol Int. 2009;58:21–28. - PubMed
-
- Sullivan BM, Locksley RM. Basophils: a nonredundant contributor to host immunity. Immunity. 2009;30:12–20. - PubMed
-
- Gauvreau GM, Ellis AK, Denburg JA. Haemopoietic processes in allergic disease: eosinophil/basophil development. Clin Exp Allergy. 2009;39:1297–1306. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

