Linking neuronal ensembles by associative synaptic plasticity
- PMID: 21738576
- PMCID: PMC3126801
- DOI: 10.1371/journal.pone.0020486
Linking neuronal ensembles by associative synaptic plasticity
Abstract
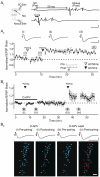
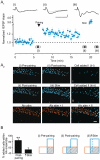
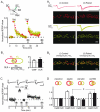
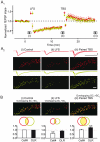
Synchronized activity in ensembles of neurons recruited by excitatory afferents is thought to contribute to the coding information in the brain. However, the mechanisms by which neuronal ensembles are generated and modified are not known. Here we show that in rat hippocampal slices associative synaptic plasticity enables ensembles of neurons to change by incorporating neurons belonging to different ensembles. Associative synaptic plasticity redistributes the composition of different ensembles recruited by distinct inputs such as to specifically increase the similarity between the ensembles. These results show that in the hippocampus, the ensemble of neurons recruited by a given afferent projection is fluid and can be rapidly and persistently modified to specifically include neurons from different ensembles. This linking of ensembles may contribute to the formation of associative memories.
Conflict of interest statement
Figures

References
-
- Averbeck BB, Latham PE, Pouget A. Neural correlations, population coding and computation. Nat Rev Neurosci. 2006;7:358–366. - PubMed
-
- Usrey WM, Reid RC. Synchronous activity in the visual system. Annu Rev Physiol. 1999;61:435–456. - PubMed
-
- Ohki K, Chung S, Ch'ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. - PubMed
-
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. - PubMed
-
- Leutgeb S, Leutgeb JK, Moser MB, Moser EI. Place cells, spatial maps and the population code for memory. Curr Opin Neurobiol. 2005;15:738–746. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

