VapC toxins from Mycobacterium tuberculosis are ribonucleases that differentially inhibit growth and are neutralized by cognate VapB antitoxins
- PMID: 21738782
- PMCID: PMC3126847
- DOI: 10.1371/journal.pone.0021738
VapC toxins from Mycobacterium tuberculosis are ribonucleases that differentially inhibit growth and are neutralized by cognate VapB antitoxins
Abstract
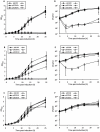
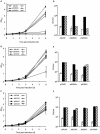
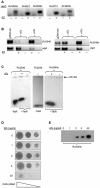
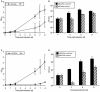
The chromosome of Mycobacterium tuberculosis (Mtb) encodes forty seven toxin-antitoxin modules belonging to the VapBC family. The role of these modules in the physiology of Mtb and the function(s) served by their expansion are unknown. We investigated ten vapBC modules from Mtb and the single vapBC from M. smegmatis. Of the Mtb vapCs assessed, only Rv0549c, Rv0595c, Rv2549c and Rv2829c were toxic when expressed from a tetracycline-regulated promoter in M. smegmatis. The same genes displayed toxicity when conditionally expressed in Mtb. Toxicity of Rv2549c in M. smegmatis correlated with the level of protein expressed, suggesting that the VapC level must exceed a threshold for toxicity to be observed. In addition, the level of Rv2456 protein induced in M. smegmatis was markedly lower than Rv2549c, which may account for the lack of toxicity of this and other VapCs scored as 'non-toxic'. The growth inhibitory effects of toxic VapCs were neutralized by expression of the cognate VapB as part of a vapBC operon or from a different chromosomal locus, while that of non-cognate antitoxins did not. These results demonstrated a specificity of interaction between VapCs and their cognate VapBs, a finding corroborated by yeast two-hybrid analyses. Deletion of selected vapC or vapBC genes did not affect mycobacterial growth in vitro, but rendered the organisms more susceptible to growth inhibition following toxic VapC expression. However, toxicity of 'non-toxic' VapCs was not unveiled in deletion mutant strains, even when the mutation eliminated the corresponding cognate VapB, presumably due to insufficient levels of VapC protein. Together with the ribonuclease (RNase) activity demonstrated for Rv0065 and Rv0617--VapC proteins with similarity to Rv0549c and Rv3320c, respectively--these results suggest that the VapBC family potentially provides an abundant source of RNase activity in Mtb, which may profoundly impact the physiology of the organism.
Conflict of interest statement
Figures




References
-
- Dhar N, McKinney JD. Microbial phenotypic heterogeneity and antibiotic tolerance. Curr Opin Microbiol. 2007;10:30–38. - PubMed
-
- Stewart GR, Robertson BD, Young DB. Tuberculosis: a problem with persistence. Nat Rev Microbiol. 2003;1:97–105. - PubMed
-
- Barry CE, 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, et al. Spectrum of latent tuberculosis - existing tests cannot resolve the underlying phenotypes: author's reply. Nat Rev Microbiol. 2010;8:242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

