Vancomycin-associated spontaneous cutaneous adverse drug reactions
- PMID: 21738885
- PMCID: PMC3121061
- DOI: 10.4168/aair.2011.3.3.194
Vancomycin-associated spontaneous cutaneous adverse drug reactions
Abstract
Purpose: With the increase in vancomycin use, adverse drug reactions (ADRs) associated with vancomycin have been reported increasingly more often. However, the characteristics of cutaneous ADRs with and without systemic reactions (SRs) have not been described. This study investigated the characteristics of spontaneously reported and assessed ADRs associated with vancomycin by a pharmacovigilance center.
Methods: ADRs (n=121) associated with vancomycin in 96 patients were collected from 2008 to 2009. Records from physician- and nurse-reported suspected cases of vancomycin ADRs, ADR type, latent period, and laboratory results were compared between cutaneous ADRs with and without SRs.
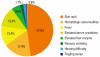
Results: The main vancomycin-related ADRs were skin rashes (47.9%), hematologic abnormalities (17.36%), fever (12.4%), and elevated serum creatinine (12.4%). Significant differences were observed in latent period (days) and the mean change in eosinophils (%) between cutaneous (9.21±9.71 and 1.4±3.4, respectively) and other ADRs (14.03±11.71 and -0.5±3.5, respectively). Twelve cases of cutaneous ADRs with SRs had been initially reported as cutaneous ADRs only. Mean changes in the eosinophil count were significantly higher for cutaneous ADRs with SRs compared to those without SRs.
Conclusions: Skin rashes accompanied by peripheral eosinophilia, representing suspected immune-mediated delayed hypersensitivity reactions, are a common vancomycin ADR. For the early and exact detection of ADRs associated with vancomycin administration, close monitoring of laboratory tests, including complete blood counts with differential analysis, is recommended.
Keywords: Vancomycin; adverse drug reaction; eosinophilia.
Conflict of interest statement
There are no financial or other issues that might lead to conflict of interest.
Figures
References
-
- Wong JT, Ripple RE, MacLean JA, Marks DR, Bloch KJ. Vancomycin hypersensitivity: synergism with narcotics and "desensitization" by a rapid continuous intravenous protocol. J Allergy Clin Immunol. 1994;94:189–194. - PubMed
-
- Levine DP. Vancomycin: a history. Clin Infect Dis. 2006;42(Suppl 1):S5–S12. - PubMed
-
- Kupstaite R, Baranauskaite A, Pileckyte M, Sveikata A, Kadusevicius E, Muckiene G. Severe vancomycin-induced anaphylactic reaction. Medicina (Kaunas) 2010;46:30–33. - PubMed
-
- Rocha JL, Kondo W, Baptista MI, Da Cunha CA, Martins LT. Uncommon vancomycin-induced side effects. Braz J Infect Dis. 2002;6:196–200. - PubMed