4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning
- PMID: 21739523
- PMCID: PMC3193906
- DOI: 10.1002/hipo.20964
4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning
Abstract
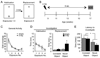
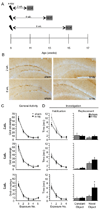
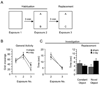
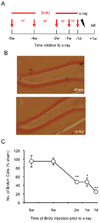
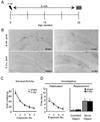
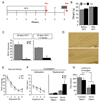
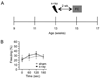
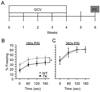
To explore the role of adult hippocampal neurogenesis in novelty processing, we assessed novel object recognition (NOR) in mice after neurogenesis was arrested using focal x-irradiation of the hippocampus, or a reversible, genetic method in which glial fibrillary acidic protein-positive neural progenitor cells are ablated with ganciclovir. Arresting neurogenesis did not alter general activity or object investigation during four exposures with two constant objects. However, when a novel object replaced a constant object, mice with neurogenesis arrested by either ablation method showed increased exploration of the novel object when compared with control mice. The increased novel object exploration did not manifest until 4-6 weeks after x-irradiation or 6 weeks following a genetic ablation, indicating that exploration of the novel object is increased specifically by the elimination of 4- to 6-week-old adult born neurons. The increased novel object exploration was also observed in older mice, which exhibited a marked reduction in neurogenesis relative to young mice. Mice with neurogenesis arrested by either ablation method were also impaired in one-trial contextual fear conditioning (CFC) at 6 weeks but not at 4 weeks following ablation, further supporting the idea that 4- to 6-week-old adult born neurons are necessary for specific forms of hippocampal-dependent learning, and suggesting that the NOR and CFC effects have a common underlying mechanism. These data suggest that the transient enhancement of plasticity observed in young adult-born neurons contributes to cognitive functions.
Copyright © 2011 Wiley Periodicals, Inc.
Figures


References
-
- Aggleton JP, Keen S, Warburton EC, Bussey TJ. Extensive cytotoxic lesions involving both the rhinal cortices and area TE impair recognition but spare spatial alternation in the rat. Brain Res Bull. 1997;43:279–287. - PubMed
-
- Gray A. The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. Oxford: Oxford University Press; 1982. p. 548.
-
- Baxter MG, Murray EA. Opposite relationship of hippocampal and rhinal cortex damage to delayed nonmatching-to-sample deficits in monkeys. Hippocampus. 2001:61–71. - PubMed
-
- Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9:562–574. - PubMed
-
- Ben Abdallah NM-B, Slomianka L, Vyssotski AL, Lipp H-P. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging. 2010;31:151–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

