An isolated cryptic peptide influences osteogenesis and bone remodeling in an adult mammalian model of digit amputation
- PMID: 21740273
- PMCID: PMC3226059
- DOI: 10.1089/ten.TEA.2011.0257
An isolated cryptic peptide influences osteogenesis and bone remodeling in an adult mammalian model of digit amputation
Abstract
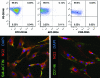
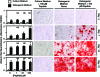
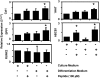
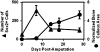
Biologic scaffolds composed of extracellular matrix (ECM) have been used successfully in preclinical models and humans for constructive remodeling of functional, site-appropriate tissue after injury. The mechanisms underlying ECM-mediated constructive remodeling are not completely understood, but scaffold degradation and site-directed recruitment of progenitor cells are thought to play critical roles. Previous studies have identified a cryptic peptide derived from the C-terminal telopeptide of collagen IIIα that has chemotactic activity for progenitor cells. The present study characterized the osteogenic activity of the same peptide in vitro and in vivo in an adult murine model of digit amputation. The present study showed that the cryptic peptide increased calcium deposition, alkaline phosphatase activity, and osteogenic gene expression in human perivascular stem cells in vitro. Treatment with the cryptic peptide in a murine model of mid-second phalanx digit amputation led to the formation of a bone nodule at the site of amputation. In addition to potential therapeutic implications for the treatment of bone injuries and facilitation of reconstructive surgical procedures, cryptic peptides with the ability to alter stem cell recruitment and differentiation at a site of injury may serve as powerful new tools for influencing stem cell fate in the local injury microenvironment.
Figures





References
-
- Caione P. Capozza N. Zavaglia D. Palombaro G. Boldrini R. In vivo bladder regeneration using small intestinal submucosa: experimental study. Pediatr Surg Int. 2006;22:593. - PubMed
-
- Cobb M.A. Badylak S.F. Janas W. Boop F.A. Histology after dural grafting with small intestinal submucosa. Surg Neurol. 1996;46:389. discussion 93–94. - PubMed
-
- Lantz G.C. Badylak S.F. Coffey A.C. Geddes L.A. Blevins W.E. Small intestinal submucosa as a small-diameter arterial graft in the dog. J Invest Surg. 1990;3:217. - PubMed
-
- Hodde J.P. Badylak S.F. Shelbourne K.D. The effect of range of motion on remodeling of small intestinal submucosa (SIS) when used as an achilles tendon repair material in the rabbit. Tissue Eng. 1997;3:27.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

