The 5-HT3B subunit affects high-potency inhibition of 5-HT3 receptors by morphine
- PMID: 21740409
- PMCID: PMC3315041
- DOI: 10.1111/j.1476-5381.2011.01582.x
The 5-HT3B subunit affects high-potency inhibition of 5-HT3 receptors by morphine
Abstract
Background and purpose: Morphine is an antagonist at 5-HT(3) A receptors. 5-HT(3) and opioid receptors are expressed in many of the same neuronal pathways where they modulate gut motility, pain and reinforcement. There is increasing interest in the 5-HT3B subunit, which confers altered pharmacology to 5-HT(3) receptors. We investigated the mechanisms of inhibition by morphine of 5-HT(3) receptors and the influence of the 5-HT3B subunit.
Experimental approach: 5-HT-evoked currents were recorded from voltage-clamped HEK293 cells expressing human 5-HT3A subunits alone or in combination with 5-HT3B subunits. The affinity of morphine for the orthosteric site of 5-HT(3) A or 5-HT(3) AB receptors was assessed using radioligand binding with the antagonist [(3) H]GR65630.
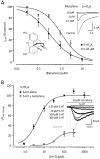
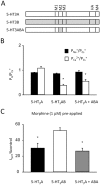
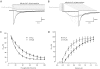
Key results: When pre-applied, morphine potently inhibited 5-HT-evoked currents mediated by 5-HT(3) A receptors. The 5-HT3B subunit reduced the potency of morphine fourfold and increased the rates of inhibition and recovery. Inhibition by pre-applied morphine was insurmountable by 5-HT, was voltage-independent and occurred through a site outside the second membrane-spanning domain. When applied simultaneously with 5-HT, morphine caused a lower potency, surmountable inhibition of 5-HT(3) A and 5-HT(3) AB receptors. Morphine also fully displaced [(3) H]GR65630 from 5-HT(3) A and 5-HT(3) AB receptors with similar potency.
Conclusions and implications: These findings suggest that morphine has two sites of action, a low-affinity, competitive site and a high-affinity, non-competitive site that is not available when the channel is activated. The affinity of morphine for the latter is reduced by the 5-HT3B subunit. Our results reveal that morphine causes a high-affinity, insurmountable and subunit-dependent inhibition of human 5-HT(3) receptors.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures


References
-
- Allan AM, Galindo R, Chynoweth J, Engel SR, Savage DD. Conditioned place preference for cocaine is attenuated in mice over-expressing the 5-HT3 receptor. Psychopharmacology (Berl) 2001;158:18–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

