SAMHD1: a new insight into HIV-1 restriction in myeloid cells
- PMID: 21740548
- PMCID: PMC3142215
- DOI: 10.1186/1742-4690-8-55
SAMHD1: a new insight into HIV-1 restriction in myeloid cells
Abstract
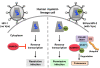
Human myeloid-lineage cells are refractory to HIV-1 infection. The Vpx proteins from HIV-2 and sooty mangabey SIV render these cells permissive to HIV-1 infection through proteasomal degradation of a putative restriction factor. Two recent studies discovered the cellular protein SAMHD1 to be this restriction factor, demonstrating that Vpx induces proteasomal degradation of SAMHD1 and enhances HIV-1 infection in myeloid-lineage cells. SAMHD1 functions as a myeloid-cell-specific HIV-1 restriction factor by inhibiting viral DNA synthesis. Here we discuss the implications of these findings in delineating the mechanisms of HIV-1 restriction in myeloid-lineage cells and the potential role of Vpx in lentiviral pathogenesis.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

