miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis
- PMID: 21741600
- PMCID: PMC3681522
- DOI: 10.1016/j.ccr.2011.05.027
miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis
Abstract
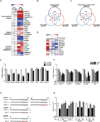
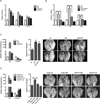
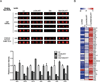
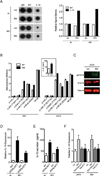
To metastasize, a tumor cell must acquire abilities such as the capacity to colonize new tissue and evade immune surveillance. Recent evidence suggests that microRNAs can promote the evolution of malignant behaviors by regulating multiple targets. We performed a microRNA analysis of human melanoma, a highly invasive cancer, and found that miR-30b/30d upregulation correlates with stage, metastatic potential, shorter time to recurrence, and reduced overall survival. Ectopic expression of miR-30b/30d promoted the metastatic behavior of melanoma cells by directly targeting the GalNAc transferase GALNT7, resulted in increased synthesis of the immunosuppressive cytokine IL-10, and reduced immune cell activation and recruitment. These data support a key role of miR-30b/30d and GalNAc transferases in metastasis, by simultaneously promoting cellular invasion and immunosuppression.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



References
-
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. - PubMed
-
- Bhavanandan VP. Cancer-associated mucins and mucin-type glycoproteins. Glycobiology. 1991;1:493–503. - PubMed
-
- Bogunovic D, O'Neill DW, Belitskaya-Levy I, Vacic V, Yu YL, Adams S, Darvishian F, Berman R, Shapiro R, Pavlick AC, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci U S A. 2009;106:20429–20434. - PMC - PubMed
-
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

