High molecular weight isoforms of growth hormone in cells of the immune system
- PMID: 21741628
- PMCID: PMC3751194
- DOI: 10.1016/j.cellimm.2011.06.001
High molecular weight isoforms of growth hormone in cells of the immune system
Abstract
A substantial body of research exists to support the idea that cells of the immune system produce growth hormone (GH). However, the structure and mechanism of action of lymphocyte-derived GH continues to remain largely unknown. Here we present the results of Western analysis of whole cell extracts showing that different molecular weight isoforms of GH of approximately 100, 65, and 48 kDa can be detected in primary mouse cells of the immune system and in the mouse EL4 cell line. The identity of the 65 and 48 kDa isoforms of GH were confirmed by mass spectrometry. The various isoforms were detected in both enriched T and B spleen cell populations. The large molecular weight isoform appears to reside primarily in the cytoplasm, whereas the lower molecular weight 65 and 48 kDa isoforms were detected primarily in the nucleus. These results also suggest that GH isoforms are induced by oxidative stress. In EL4 cells overexpressing GH, the expression of luciferase controlled by a promoter containing the antioxidant response element is increased almost threefold above control. The data suggest that the induction of isoforms of the GH molecule in cells of the immune system may be an important mechanism of adaptation and/or protection of lymphoid cells under conditions of oxidative stress.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
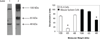
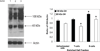




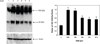

Similar articles
-
Hypoxia and cytoplasmic alkalinization upregulate growth hormone expression in lymphocytes.Cell Immunol. 2013 Mar;282(1):9-16. doi: 10.1016/j.cellimm.2013.03.007. Epub 2013 Apr 11. Cell Immunol. 2013. PMID: 23639351 Free PMC article.
-
The production of nitric oxide in EL4 lymphoma cells overexpressing growth hormone.J Neuroimmunol. 2003 Jan;134(1-2):82-94. doi: 10.1016/s0165-5728(02)00420-4. J Neuroimmunol. 2003. PMID: 12507775
-
Growth hormone prevents Fas-induced apoptosis in lymphocytes through modulation of Bcl-2 and caspase-3.Neuroimmunomodulation. 2001;9(5):256-62. doi: 10.1159/000054288. Neuroimmunomodulation. 2001. PMID: 11964520
-
Effect of growth hormone (GH) on the immune system.Pediatr Endocrinol Rev. 2004 Aug;1 Suppl 3:490-5. Pediatr Endocrinol Rev. 2004. PMID: 16444180 Review.
-
Lymphocyte GH-axis hormones in immunity.Cell Immunol. 2013 Sep-Oct;285(1-2):118-32. doi: 10.1016/j.cellimm.2013.10.003. Epub 2013 Oct 19. Cell Immunol. 2013. PMID: 24177252 Review.
Cited by
-
Detecting suicide risk in bipolar disorder patients from lymphoblastoid cell lines genetic signatures.Transl Psychiatry. 2025 Sep 3;15(1):339. doi: 10.1038/s41398-025-03573-3. Transl Psychiatry. 2025. PMID: 40903457 Free PMC article.
-
Non-pituitary GH regulation of the tissue microenvironment.Endocr Relat Cancer. 2023 Jun 1;30(7):e230028. doi: 10.1530/ERC-23-0028. Print 2023 Jul 1. Endocr Relat Cancer. 2023. PMID: 37066857 Free PMC article.
-
Growth hormone therapy in pediatric kidney transplantation-the long-term clinical benefits beyond improvement of growth after withdrawal of pre-transplant therapy.Pediatr Nephrol. 2022 Apr;37(4):699-702. doi: 10.1007/s00467-021-05223-4. Epub 2021 Sep 20. Pediatr Nephrol. 2022. PMID: 34542702 No abstract available.
-
Upregulation of GH, but not IGF1, in the hippocampus of the lactating dam after kainic acid injury.Endocr Connect. 2018 Feb;7(2):258-267. doi: 10.1530/EC-17-0380. Epub 2018 Jan 10. Endocr Connect. 2018. PMID: 29321175 Free PMC article.
-
Expression of lymphocyte-derived growth hormone (GH) and GH-releasing hormone receptors in aging rats.Cell Immunol. 2013 Apr;282(2):71-8. doi: 10.1016/j.cellimm.2013.04.009. Epub 2013 May 3. Cell Immunol. 2013. PMID: 23770714 Free PMC article.
References
-
- Maggiano N, Piantelli M, Ricci R, Larocca LM, Capelli A, Ranelletti O. Detection of growth hormone-producing cells in human thymus by immunohistochemistry and non-radioactive in situ hybridization. J Histochem Cytochem. 1994;42:1349–1354. - PubMed
-
- Wu H, Devi R, Malarkey WB. Localization of the growth hormone messenger ribonucleic acid in the human immune system--a clinical research center study. J Clin Endocrinol Metab. 1996;81:1278–1282. - PubMed
-
- Palmetshofer A, Zechner D, Luger TA, Barta A. Splicing variants of the human growth hormone mRNA: detection in pituitary, mononuclear cells and dermal fibroblasts. Mol Cell Endocrinol. 1995;113:225–234. - PubMed
-
- Weigent DA, Blalock JE. The production of growth hormone by subpopulations of rat mononuclear leukocytes. Cell Immunol. 1991;135:55–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

