The tripeptide KdPT protects from intestinal inflammation and maintains intestinal barrier function
- PMID: 21741932
- PMCID: PMC3157275
- DOI: 10.1016/j.ajpath.2011.05.013
The tripeptide KdPT protects from intestinal inflammation and maintains intestinal barrier function
Abstract
Treatment options for inflammatory bowel disease (IBD) are incompletely helpful, and surgery is often needed. One promising class of future therapeutic agents for IBD is melanocortin-related peptides, which exhibit potent immunomodulatory effects. We investigated KdPT, a tripeptide derivative of the C-terminus of α-melanocyte-stimulating hormone, as an anti-inflammatory small molecule in vivo and in vitro. Intestinal inflammation was studied after oral administration of dextran sodium sulfate and in IL-10 gene-deficient mice. The effects of KdPT on key colonic epithelial cell functions were studied in vitro and in vivo by evaluating proliferation, wound healing, transepithelial resistance, and expression of tight junction proteins. Melanin assays were performed to determine the melanotropic effects of KdPT. KdPT-treated animals showed markedly reduced severity of inflammation in both colitis models. In colonic epithelial cells, KdPT increased proliferation, accelerated closure of wounds, and improved transepithelial electrical resistance after stimulation with interferon-γ/tumor necrosis factor-α. Moreover, treatment with KdPT also prevented the loss of tight junction protein expression and improved barrier function in vivo. KdPT acted independently of IL-1 receptor type I in vivo and did not affect melanogenesis in vitro. KdPT is capable of attenuating the course of experimental colitis in different models and maintains epithelial cell function. Furthermore, KdPT does not induce pigmentation, emphasizing the potential of this small molecule for the future treatment of IBD.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

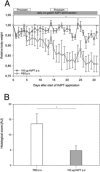








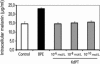
References
-
- Bousvaros A., Sylvester F., Kugathasan S., Szigethy E., Fiocchi C., Colletti R., Otley A., Amre D., Ferry G., Czinn S.J., Splawski J.B., Oliva-Hemker M., Hyams J.S., Faubion W.A., Kirschner B.S., Dubinsky M.C. Challenges in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:885–913. - PubMed
-
- Papadakis K.A., Targan S.R. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289–298. - PubMed
-
- Hoffmann J.C., Preiss J.C., Autschbach F., Buhr H.J., Hauser W., Herrlinger K., Hohne W., Koletzko S., Krieglstein C.F., Kruis W., Matthes H., Moser G., Reinshagen M., Rogler G., Schreiber S., Schreyer A.G., Sido B., Siegmund B., Stallmach A., Bokemeyer B., Stange E.F., Zeitz M. [Clinical practice guideline on diagnosis and treatment of Crohn's disease]: German. Z Gastroenterol. 2008;46:1094–1146. - PubMed
-
- Leowardi C., Heuschen G., Kienle P., Heuschen U., Schmidt J. Surgical treatment of severe inflammatory bowel diseases. Dig Dis. 2003;21:54–62. - PubMed
-
- Gionchetti P., Rizzello F., Helwig U., Venturi A., Lammers K.M., Brigidi P., Vitali B., Poggioli G., Miglioli M., Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–1209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

