Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation
- PMID: 21741942
- PMCID: PMC3157227
- DOI: 10.1016/j.ajpath.2011.05.031
Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation
Abstract
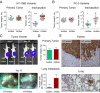
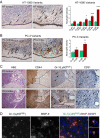
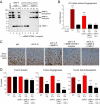
Tumor-associated neutrophils contribute to neovascularization by supplying matrix metalloproteinase-9 (MMP-9), a protease that has been genetically and biochemically linked to induction of angiogenesis. Specific roles of inflammatory neutrophils and their distinct proMMP-9 in the coordinate regulation of tumor angiogenesis and tumor cell dissemination, however, have not been addressed. We demonstrate that the primary tumors formed by highly disseminating variants of human fibrosarcoma and prostate carcinoma recruit elevated levels of infiltrating MMP-9-positive neutrophils and concomitantly exhibit enhanced levels of angiogenesis and intravasation. Specific inhibition of neutrophil influx by interleukin 8 (IL-8) neutralization resulted in the coordinated diminishment of tumor angiogenesis and intravasation, both of which were rescued by purified neutrophil proMMP-9. However, if neutrophil proMMP-9, naturally devoid of tissue inhibitor of metalloproteinases (TIMP), was delivered in complex with TIMP-1 or in a mixture with TIMP-2, the protease failed to rescue the inhibitory effects of anti-IL8 therapy, indicating that the TIMP-free status of proMMP-9 is critical for facilitating tumor angiogenesis and intravasation. Our findings directly link tumor-associated neutrophils and their TIMP-free proMMP-9 with the ability of aggressive tumor cells to induce the formation of new blood vessels that serve as conduits for tumor cell dissemination. Thus, treatment of cancers associated with neutrophil infiltration may benefit from specific targeting of neutrophil MMP-9 at early stages to prevent ensuing tumor angiogenesis and tumor metastasis.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

