The G protein-coupled estrogen receptor GPER/GPR30 as a regulator of cardiovascular function
- PMID: 21742056
- PMCID: PMC3216677
- DOI: 10.1016/j.vph.2011.06.003
The G protein-coupled estrogen receptor GPER/GPR30 as a regulator of cardiovascular function
Abstract
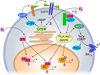
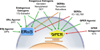
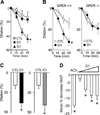
Endogenous estrogens are important regulators of cardiovascular homeostasis in premenopausal women and delay the development of hypertension and coronary artery disease. These hormones act via three different estrogen receptors affecting both gene transcription and rapid signaling pathways in a complex interplay. In addition to the classical estrogen receptors ERα and ERβ, which are known mediators of estrogen-dependent vascular effects, a G protein-coupled estrogen receptor termed GPER that is expressed in the cardiovascular system has recently been identified. Endogenous human 17β-estradiol, selective estrogen receptor modulators (SERMs) including tamoxifen and raloxifene, and selective estrogen receptor downregulators (SERDs) such as ICI 182,780 are all agonists of GPER, which has been implicated in the regulation of vasomotor tone and protection from myocardial ischemia/reperfusion injury. As a result, understanding the individual role of ERα, ERβ, and GPER in cardiovascular function has become increasingly complex. With accumulating evidence that GPER is responsible for a variety of beneficial cardiovascular effects of estrogens, this receptor may represent a novel target to develop effective strategies for the treatment of cardiovascular diseases by tissue-specific, selective activation of estrogen-dependent molecular pathways devoid of side effects seen with conventional hormone therapy.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Estrogens and Coronary Artery Disease: New Clinical Perspectives.Adv Pharmacol. 2016;77:307-60. doi: 10.1016/bs.apha.2016.05.003. Epub 2016 Jun 29. Adv Pharmacol. 2016. PMID: 27451102 Review.
-
Estrogen-mediated inactivation of FOXO3a by the G protein-coupled estrogen receptor GPER.BMC Cancer. 2015 Oct 15;15:702. doi: 10.1186/s12885-015-1699-6. BMC Cancer. 2015. PMID: 26470790 Free PMC article.
-
Reciprocality Between Estrogen Biology and Calcium Signaling in the Cardiovascular System.Front Endocrinol (Lausanne). 2020 Sep 29;11:568203. doi: 10.3389/fendo.2020.568203. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33133016 Free PMC article. Review.
-
Not lost in translation: Emerging clinical importance of the G protein-coupled estrogen receptor GPER.Steroids. 2016 Jul;111:37-45. doi: 10.1016/j.steroids.2016.02.016. Epub 2016 Feb 24. Steroids. 2016. PMID: 26921679 Review.
-
GPER: a novel target for non-genomic estrogen action in the cardiovascular system.Pharmacol Res. 2013 May;71:53-60. doi: 10.1016/j.phrs.2013.02.008. Epub 2013 Mar 4. Pharmacol Res. 2013. PMID: 23466742 Review.
Cited by
-
Emerging roles of GPER in diabetes and atherosclerosis.Trends Endocrinol Metab. 2015 Apr;26(4):185-92. doi: 10.1016/j.tem.2015.02.003. Epub 2015 Mar 9. Trends Endocrinol Metab. 2015. PMID: 25767029 Free PMC article. Review.
-
Pregnancy Augments G Protein Estrogen Receptor (GPER) Induced Vasodilation in Rat Uterine Arteries via the Nitric Oxide - cGMP Signaling Pathway.PLoS One. 2015 Nov 4;10(11):e0141997. doi: 10.1371/journal.pone.0141997. eCollection 2015. PLoS One. 2015. PMID: 26536245 Free PMC article.
-
A single dose of estrogen during hemorrhagic shock protects against Kidney Injury whereas estrogen restoration in ovariectomized mice is ineffective.Sci Rep. 2020 Oct 14;10(1):17240. doi: 10.1038/s41598-020-73974-5. Sci Rep. 2020. PMID: 33057080 Free PMC article.
-
GPER-novel membrane oestrogen receptor.Clin Sci (Lond). 2016 Jun 1;130(12):1005-16. doi: 10.1042/CS20160114. Clin Sci (Lond). 2016. PMID: 27154744 Free PMC article. Review.
-
Improvement of vascular function by acute and chronic treatment with the GPR30 agonist G1 in experimental diabetes mellitus.PLoS One. 2012;7(6):e38787. doi: 10.1371/journal.pone.0038787. Epub 2012 Jun 5. PLoS One. 2012. PMID: 22679517 Free PMC article.
References
-
- Al Zubair K, Razak A, Bexis S, Docherty JR. Relaxations to oestrogen receptor subtype selective agonists in rat and mouse arteries. Eur J Pharmacol. 2005;513:101–108. - PubMed
-
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Ando S, Maggiolini M. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–1866. - PubMed
-
- Babiker FA, Lips DJ, Delvaux E, Zandberg P, Janssen BJ, Prinzen F, van Eys G, Grohe C, Doevendans PA. Oestrogen modulates cardiac ischaemic remodelling through oestrogen receptor-specific mechanisms. Acta Physiol (Oxf) 2007;189:23–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

