Surfactant therapy for acute lung injury and acute respiratory distress syndrome
- PMID: 21742216
- PMCID: PMC3153076
- DOI: 10.1016/j.ccc.2011.04.005
Surfactant therapy for acute lung injury and acute respiratory distress syndrome
Abstract
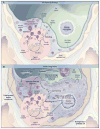
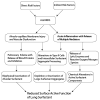
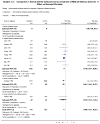
This article examines exogenous lung surfactant replacement therapy and its usefulness in mitigating clinical acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS). Surfactant therapy is beneficial in term infants with pneumonia and meconium aspiration lung injury, and in children up to age 21 years with direct pulmonary forms of ALI/ARDS. However, extension of exogenous surfactant therapy to adults with respiratory failure and clinical ALI/ARDS remains a challenge. This article reviews clinical studies of surfactant therapy in pediatric and adult patients with ALI/ARDS, focusing on its potential advantages in patients with direct pulmonary forms of these syndromes.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Surfactant for pediatric acute lung injury.Pediatr Clin North Am. 2008 Jun;55(3):545-75, ix. doi: 10.1016/j.pcl.2008.02.016. Pediatr Clin North Am. 2008. PMID: 18501754 Free PMC article. Review.
-
The future of exogenous surfactant therapy.Respir Care. 2011 Sep;56(9):1369-86; discussion 1386-8. doi: 10.4187/respcare.01306. Respir Care. 2011. PMID: 21944686 Review.
-
The role of surfactant treatment in preterm infants and term newborns with acute respiratory distress syndrome.J Perinatol. 2009 May;29 Suppl 2:S18-22. doi: 10.1038/jp.2009.30. J Perinatol. 2009. PMID: 19399004 Review.
-
Computed tomography assessment of exogenous surfactant-induced lung reaeration in patients with acute lung injury.Crit Care. 2010;14(4):R135. doi: 10.1186/cc9186. Epub 2010 Jul 15. Crit Care. 2010. PMID: 20633284 Free PMC article. Clinical Trial.
-
Exogenous surfactant in paediatric Acute Lung Injury and Acute Respiratory Distress Syndrome.Curr Drug Saf. 2006 May;1(2):159-68. doi: 10.2174/157488606776930553. Curr Drug Saf. 2006. PMID: 18690927 Review.
Cited by
-
Increased phospholipase A2 and lyso-phosphatidylcholine levels are associated with surfactant dysfunction in lung contusion injury in mice.Surgery. 2013 Jan;153(1):25-35. doi: 10.1016/j.surg.2012.05.043. Epub 2012 Jul 31. Surgery. 2013. PMID: 22853859 Free PMC article.
-
The Use of Exogenous Lung Surfactant (Poractant Alfa) in Acute Respiratory Failure by Drowning.Case Rep Crit Care. 2020 Jun 6;2020:9270791. doi: 10.1155/2020/9270791. eCollection 2020. Case Rep Crit Care. 2020. PMID: 32566323 Free PMC article.
-
Paracrine factors from mesenchymal stem cells: a proposed therapeutic tool for acute lung injury and acute respiratory distress syndrome.Int Wound J. 2014 Apr;11(2):114-21. doi: 10.1111/iwj.12202. Epub 2013 Dec 26. Int Wound J. 2014. PMID: 24373614 Free PMC article. Review.
-
Activity and biophysical inhibition resistance of a novel synthetic lung surfactant containing Super-Mini-B DATK peptide.PeerJ. 2016 Jan 5;4:e1528. doi: 10.7717/peerj.1528. eCollection 2016. PeerJ. 2016. PMID: 26793419 Free PMC article.
-
Synergistic Therapeutic Effects of D-Mannitol-Cerium-Quercetin (Rutin) Coordination Polymer Nanoparticles on Acute Lung Injury.Molecules. 2024 Jun 13;29(12):2819. doi: 10.3390/molecules29122819. Molecules. 2024. PMID: 38930884 Free PMC article.
References
-
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS: Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. - PubMed
-
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. - PubMed
-
- American College of Chest Physicians Society of Critical Care Medicine Consensus Conference Committee. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies for sepsis. Crit Care Med. 1992;20:864–874. - PubMed
-
- Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated pediatric risk of mortality score. Crit Care Med. 1996;24:743–752. - PubMed
-
- Slater A, Shann F ANZICS Paediatric Study Group. The suitability of the Pediatric Index of Mortality (PIM), PIM2, the Pediatric Risk of Mortality (PRISM), and PRISM III for monitoring the quality of pediatric intensive care in Australia and New Zealand. Pediatr Crit Care Med. 2004;5:447–454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

