Osteopontin upregulation in rotavirus-induced murine biliary atresia requires replicating virus but is not necessary for development of biliary atresia
- PMID: 21742364
- PMCID: PMC3170515
- DOI: 10.1016/j.virol.2011.05.015
Osteopontin upregulation in rotavirus-induced murine biliary atresia requires replicating virus but is not necessary for development of biliary atresia
Abstract
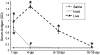
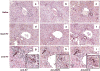
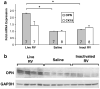
Biliary atresia (BA) is a progressive fibro-inflammatory pediatric liver disease in which osteopontin (OPN), a glycoprotein with inflammatory and fibrogenic activity, may play a pathogenic role. The current studies were conducted in a mouse model of rotavirus-induced BA to test the hypotheses that live but not inactivated rotavirus causes antigenemia, upregulation of hepatic OPN expression, and induction of BA and fibrosis; and that OPN is necessary for development of BA. Prolonged or transient antigenemia developed in mice inoculated with live or inactivated virus, respectively, but only live virus upregulated hepatic OPN and caused BA and fibrosis. OPN was expressed in intra- and extrahepatic bile ducts in healthy mice and in mice with BA. OPN-deficient mice, similar to WT mice, developed BA. Together, these data show that live but not inactivated rotavirus causes upregulation of hepatic OPN expression and BA but that OPN is not necessary for development of BA.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
The Role of Neonatal Gr-1+ Myeloid Cells in a Murine Model of Rhesus-Rotavirus-Induced Biliary Atresia.Am J Pathol. 2018 Nov;188(11):2617-2628. doi: 10.1016/j.ajpath.2018.07.024. Epub 2018 Sep 8. Am J Pathol. 2018. PMID: 30201498
-
Role of myeloid differentiation factor 88 in Rhesus rotavirus-induced biliary atresia.J Surg Res. 2013 Sep;184(1):322-9. doi: 10.1016/j.jss.2013.05.032. Epub 2013 Jun 1. J Surg Res. 2013. PMID: 23768919 Free PMC article.
-
A Mouse Model of Chronic Liver Fibrosis for the Study of Biliary Atresia.J Vis Exp. 2023 Feb 3;(192). doi: 10.3791/65044. J Vis Exp. 2023. PMID: 36807296
-
Innate Immunity and Pathogenesis of Biliary Atresia.Front Immunol. 2020 Feb 25;11:329. doi: 10.3389/fimmu.2020.00329. eCollection 2020. Front Immunol. 2020. PMID: 32161597 Free PMC article. Review.
-
Recent progress in the etiopathogenesis of pediatric biliary disease, particularly Caroli's disease with congenital hepatic fibrosis and biliary atresia.Histol Histopathol. 2010 Feb;25(2):223-35. doi: 10.14670/HH-25.223. Histol Histopathol. 2010. PMID: 20017109 Review.
Cited by
-
Rotavirus Infections: Pathophysiology, Symptoms, and Vaccination.Pathogens. 2025 May 14;14(5):480. doi: 10.3390/pathogens14050480. Pathogens. 2025. PMID: 40430800 Free PMC article. Review.
-
Rotavirus and biliary atresia: can causation be proven?Curr Opin Gastroenterol. 2012 Jan;28(1):10-7. doi: 10.1097/MOG.0b013e32834c7ae4. Curr Opin Gastroenterol. 2012. PMID: 22123643 Free PMC article. Review.
-
Osteopontin Takes Center Stage in Chronic Liver Disease.Hepatology. 2021 Apr;73(4):1594-1608. doi: 10.1002/hep.31582. Hepatology. 2021. PMID: 32986864 Free PMC article. Review.
-
Immune-mediated cholangiopathies in children: the need to better understand the pathophysiology for finding the future possible treatment targets.Front Immunol. 2023 Oct 20;14:1206025. doi: 10.3389/fimmu.2023.1206025. eCollection 2023. Front Immunol. 2023. PMID: 37928553 Free PMC article. Review.
-
Gene expression signature for biliary atresia and a role for interleukin-8 in pathogenesis of experimental disease.Hepatology. 2014 Jul;60(1):211-23. doi: 10.1002/hep.27045. Epub 2014 May 27. Hepatology. 2014. PMID: 24493287 Free PMC article. Clinical Trial.
References
-
- Bezerra JA, Tiao G, Ryckman FC, Alonson M, Sabla GE, Shneider B, Sokol RJ, Aronow BJ. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet. 2002;360:1653–1659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

