Receptor-targeted iron oxide nanoparticles for molecular MR imaging of inflamed atherosclerotic plaques
- PMID: 21742374
- PMCID: PMC3148412
- DOI: 10.1016/j.biomaterials.2011.06.026
Receptor-targeted iron oxide nanoparticles for molecular MR imaging of inflamed atherosclerotic plaques
Abstract
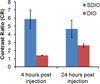
In a number of literature reports iron oxide nanoparticles have been investigated for use in imaging atherosclerotic plaques and found to accumulate in plaques via uptake by macrophages, which are critical in the process of atheroma initiation, propagation, and rupture. However, the uptake of these agents is non-specific; thus the labeling efficiency for plaques in vivo is not ideal. We have developed targeted agents to improve the efficiency for labeling macrophage-laden plaques. These probes are based on iron oxide nanoparticles coated with dextran sulfate, a ligand of macrophage scavenger receptor type A (SR-A). We have sulfated dextran-coated iron oxide nanoparticles (DIO) with sulfur trioxide, thereby targeting our nanoparticle imaging agents to SR-A. The sulfated DIO (SDIO) remained mono-dispersed and had an average hydrodynamic diameter of 62 nm, an r(1) relaxivity of 18.1 mM(-1) s(-1), and an r(2) relaxivity of 95.8 mM(-1) s(-1) (37 °C, 1.4 T). Cell studies confirmed that these nanoparticles were nontoxic and specifically targeted to macrophages. In vivo MRI after intravenous injection of the contrast agent into an atherosclerotic mouse injury model showed substantial signal loss on the injured carotid at 4 and 24 h post-injection of SDIO. No discernable signal decrease was seen at the control carotid and only mild signal loss was observed for the injured carotid post-injection of non-sulfated DIO, indicating preferential uptake of the SDIO particles at the site of atherosclerotic plaque. These results indicate that SDIO can facilitate MRI detection and diagnosis of vulnerable plaques in atherosclerosis.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
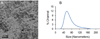

 ) or DIO (
) or DIO ( ) of different iron concentrations.
) of different iron concentrations.
 ), or dextran (
), or dextran ( ) by P388D1 cells.
) by P388D1 cells.
 ), or 24-h (
), or 24-h ( ) incubation with different concentrations of SDIO.
) incubation with different concentrations of SDIO.

References
-
- Fuster V, Lois F, Franco M. Early identification of atherosclerotic disease by noninvasive imaging. Nat Rev Cardiol. 2010;7:327–333. - PubMed
-
- Wickline SA, Neubauer AM, Winter PM, Caruthers SD, Lanza GM. Molecular imaging and therapy of atherosclerosis with targeted nanoparticles. J Magn Reson Imaging. 2007;25:667–680. - PubMed
-
- Cyrus T, Lanza GM, Wickline SA. Molecular imaging by cardiovascular MR. J Cardiovasc Magn Reson. 2007;9:827–843. - PubMed
-
- Shaw SY. Molecular imaging in cardiovascular disease: targets and opportunities. Nat Rev Cardiol. 2009;6:569–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

