Chelation of lysosomal iron protects dopaminergic SH-SY5Y neuroblastoma cells from hydrogen peroxide toxicity by precluding autophagy and Akt dephosphorylation
- PMID: 21742779
- PMCID: PMC3218672
- DOI: 10.1093/toxsci/kfr179
Chelation of lysosomal iron protects dopaminergic SH-SY5Y neuroblastoma cells from hydrogen peroxide toxicity by precluding autophagy and Akt dephosphorylation
Abstract
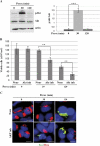
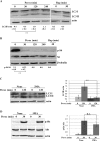
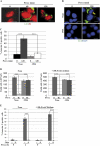
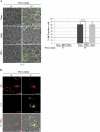
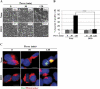
In human neuroblastoma SH-SY5Y cells, hydrogen peroxide (H(2)O(2), 200μM) rapidly (< 5 min) induced autophagy, as shown by processing and vacuolar relocation of light chain 3(LC3). Accumulation of autophagosome peaked at 30 min of H(2)O(2) exposure. The continuous presence of H(2)O(2) eventually (at > 60 min) caused autophagy-dependent annexin V-positive cell death. However, the cells exposed to H(2)O(2) for 30 min and then cultivated in fresh medium could recover and grow, despite ongoing autophagy. H(2)O(2) rapidly (5 min) triggered the formation of dichlorofluorescein-sensitive HO(·)-free radicals within mitochondria, whereas the mitochondria-associated oxidoradicals revealed by MitoSox (O(2)(·-)) became apparent after 30 min of exposure to H(2)O(2). 3-Methyladenine inhibited autophagy and cell death, but not the generation of HO(·). Genetic silencing of beclin-1 prevented bax- and annexin V-positive cell death induced by H(2)O(2), confirming the involvement of canonical autophagy in peroxide toxicity. The lysosomotropic iron chelator deferoxamine (DFO) prevented the mitochondrial generation of both HO(.) and O(2)(·-) and suppressed the induction of autophagy and of cell death by H(2)O(2). Upon exposure to H(2)O(2), Akt was intensely phosphorylated in the first 30 min, concurrently with mammalian target of rapamycin inactivation and autophagy, and it was dephosphorylated at 2 h, when > 50% of the cells were dead. DFO did not impede Akt phosphorylation, which therefore was independent of reactive oxygen species (ROS) generation but inhibited Akt dephosphorylation. In conclusion, exogenous H(2)O(2) triggers two parallel independent pathways, one leading to autophagy and autophagy-dependent apoptosis, the other to transient Akt phosphorylation, and both are inhibited by DFO. The present work establishes HO(·) as the autophagy-inducing ROS and highlights the need for free lysosomal iron for its production within mitochondria in response to hydrogen peroxide.
Figures








References
-
- Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, Ogier-Denis E. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J. Biol. Chem. 2001;276:35243–35246. - PubMed
-
- Arsham AM, Neufeld TP. Thinking globally and acting locally with TOR. Curr. Opin. Cell. Biol. 2006;18:589–597. - PubMed
-
- Bilski P, Belanger AG, Chignell CF. Photosensitized oxidation of 2′,7′-dichlorofluorescin: Singlet oxygen does not contribute to the formation of fluorescent oxidation product 2′,7′-dichlorofluorescein. Free Radic. Biol. Med. 2002;33:938–946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

