Normalization of the vasculature for treatment of cancer and other diseases
- PMID: 21742796
- PMCID: PMC3258432
- DOI: 10.1152/physrev.00038.2010
Normalization of the vasculature for treatment of cancer and other diseases
Erratum in
- Physiol Rev. 2014 Apr;94(2):707
Abstract
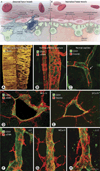
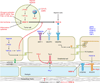
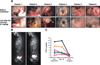
New vessel formation (angiogenesis) is an essential physiological process for embryologic development, normal growth, and tissue repair. Angiogenesis is tightly regulated at the molecular level. Dysregulation of angiogenesis occurs in various pathologies and is one of the hallmarks of cancer. The imbalance of pro- and anti-angiogenic signaling within tumors creates an abnormal vascular network that is characterized by dilated, tortuous, and hyperpermeable vessels. The physiological consequences of these vascular abnormalities include temporal and spatial heterogeneity in tumor blood flow and oxygenation and increased tumor interstitial fluid pressure. These abnormalities and the resultant microenvironment fuel tumor progression, and also lead to a reduction in the efficacy of chemotherapy, radiotherapy, and immunotherapy. With the discovery of vascular endothelial growth factor (VEGF) as a major driver of tumor angiogenesis, efforts have focused on novel therapeutics aimed at inhibiting VEGF activity, with the goal of regressing tumors by starvation. Unfortunately, clinical trials of anti-VEGF monotherapy in patients with solid tumors have been largely negative. Intriguingly, the combination of anti-VEGF therapy with conventional chemotherapy has improved survival in cancer patients compared with chemotherapy alone. These seemingly paradoxical results could be explained by a "normalization" of the tumor vasculature by anti-VEGF therapy. Preclinical studies have shown that anti-VEGF therapy changes tumor vasculature towards a more "mature" or "normal" phenotype. This "vascular normalization" is characterized by attenuation of hyperpermeability, increased vascular pericyte coverage, a more normal basement membrane, and a resultant reduction in tumor hypoxia and interstitial fluid pressure. These in turn can lead to an improvement in the metabolic profile of the tumor microenvironment, the delivery and efficacy of exogenously administered therapeutics, the efficacy of radiotherapy and of effector immune cells, and a reduction in number of metastatic cells shed by tumors into circulation in mice. These findings are consistent with data from clinical trials of anti-VEGF agents in patients with various solid tumors. More recently, genetic and pharmacological approaches have begun to unravel some other key regulators of vascular normalization such as proteins that regulate tissue oxygen sensing (PHD2) and vessel maturation (PDGFRβ, RGS5, Ang1/2, TGF-β). Here, we review the pathophysiology of tumor angiogenesis, the molecular underpinnings and functional consequences of vascular normalization, and the implications for treatment of cancer and nonmalignant diseases.
Figures








References
-
- Ader I, Delmas C, Bonnet J, Rochaix P, Favre G, Toulas C, Cohen-Jonathan-Moyal E. Inhibition of Rho pathways induces radiosensitization and oxygenation in human glioblastoma xenografts. Oncogene. 2003;22:8861–8869. - PubMed
-
- Algire GH, Chalkley HW. Vascular reactions of normal and malignant tissues in vivo. I. Vascular reactions of mice to wounds and to normal and neoplastic transplants. J Natl Cancer Inst. 1945;6:73–85.
-
- Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, Atkins JN, Seay TE, Fehrenbacher L, Goldberg RM, O’Reilly S, Chu L, Azar CA, Lopa S, Wolmark N. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP Protocol C-08. J Clin Oncol. 2011;29:11–16. - PMC - PubMed
-
- Allgayer H, Babic R, Gruetzner KU, Tarabichi A, Schildberg FW, Heiss MM. c-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J Clin Oncol. 2000;18:2201–2209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 CA073479/CA/NCI NIH HHS/United States
- R01 CA126642/CA/NCI NIH HHS/United States
- R01-CA085140/CA/NCI NIH HHS/United States
- R35 CA056591/CA/NCI NIH HHS/United States
- R01 CA085140/CA/NCI NIH HHS/United States
- R21 CA139168/CA/NCI NIH HHS/United States
- R01-CA115767/CA/NCI NIH HHS/United States
- P01-CA080124/CA/NCI NIH HHS/United States
- R24 CA085140/CA/NCI NIH HHS/United States
- R01-CA096915/CA/NCI NIH HHS/United States
- R01 CA159258/CA/NCI NIH HHS/United States
- R01 CA115767/CA/NCI NIH HHS/United States
- R01 CA096915/CA/NCI NIH HHS/United States
- P01 CA080124/CA/NCI NIH HHS/United States
- R21-CA139168/CA/NCI NIH HHS/United States
- R01-CA126642/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

