Modeling of pathological traits in Alzheimer's disease based on systemic extracellular signaling proteome
- PMID: 21742799
- PMCID: PMC3205866
- DOI: 10.1074/mcp.M111.008862
Modeling of pathological traits in Alzheimer's disease based on systemic extracellular signaling proteome
Abstract
The study of chronic brain diseases including Alzheimer's disease in patients is typically limited to brain imaging or psychometric testing. Given the epidemic rise and insufficient knowledge about pathological pathways in sporadic Alzheimer's disease, new tools are required to identify the molecular changes underlying this disease. We hypothesize that levels of specific secreted cellular signaling proteins in cerebrospinal fluid or plasma correlate with pathological changes in the Alzheimer's disease brain and can thus be used to discover signaling pathways altered in the disease. Here we measured 91 proteins of this subset of the cellular communication proteome in plasma or cerebrospinal fluid in patients with Alzheimer's disease and cognitively normal controls to mathematically model disease-specific molecular traits. We found small numbers of signaling proteins that were able to model key pathological markers of Alzheimer's disease, including levels of cerebrospinal fluid β-amyloid and tau, and classify disease in independent samples. Several of these factors had previously been implicated in Alzheimer's disease supporting the validity of our approach. Our study also points to proteins which were previously unknown to be associated with Alzheimer's disease thereby implicating novel signaling pathways in this disorder.
Conflict of interest statement
Figures
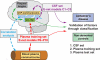

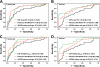
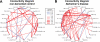
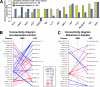
References
-
- Perry V. H., Cunningham C., Holmes C. (2007) Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 7, 161–167 - PubMed
-
- Alzheimer's Association National Office (2011) 2011 Alzheimer's Disease Facts and Figures. Alzheimer's Association National Office, IL - PubMed
-
- Dubois B., Feldman H. H., Jacova C., Cummings J. L., Dekosky S. T., Barberger-Gateau P., Delacourte A., Frisoni G., Fox N. C., Galasko D., Gauthier S., Hampel H., Jicha G. A., Meguro K., O'Brien J., Pasquier F., Robert P., Rossor M., Salloway S., Sarazin M., de Souza L. C., Stern Y., Visser P. J., Scheltens P. (2010) Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 9, 1118–1127 - PubMed
-
- Dubois B., Feldman H. H., Jacova C., Dekosky S. T., Barberger-Gateau P., Cummings J., Delacourte A., Galasko D., Gauthier S., Jicha G., Meguro K., O'brien J., Pasquier F., Robert P., Rossor M., Salloway S., Stern Y., Visser P. J., Scheltens P. (2007) Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

