Heparan sulfate regulates intraretinal axon pathfinding by retinal ganglion cells
- PMID: 21743013
- PMCID: PMC3176022
- DOI: 10.1167/iovs.11-7559
Heparan sulfate regulates intraretinal axon pathfinding by retinal ganglion cells
Abstract
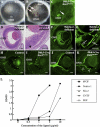
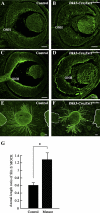
PURPOSE. Heparan sulfate (HS) is abundantly expressed in the developing neural retina; however, its role in the intraretinal axon guidance of retinal ganglion cells (RGCs) remains unclear. In this study, the authors examined whether HS was essential for the axon guidance of RGCs toward the optic nerve head. METHODS. The authors conditionally ablated the gene encoding the exostosin-1 (Ext1) enzyme, using the dickkopf homolog 3 (Dkk3)-Cre transgene, which disrupted HS expression in the mouse retina during directed pathfinding by RGC axons toward the optic nerve head. In situ hybridization, immunohistochemistry, DiI tracing, binding assay, and retinal explant assays were performed to evaluate the phenotypes of the mutants and the roles of HS in intraretinal axon guidance. RESULTS. Despite no gross abnormality in RGC distribution, the mutant RGC axons exhibited severe intraretinal guidance errors, including optic nerve hypoplasia, ectopic axon penetration through the full thickness of the neural retina and into the subretinal space, and disturbance of the centrifugal projection of RGC axons toward the optic nerve head. These abnormal phenotypes shared similarities with the RGC axon misguidance caused by mutations of genes encoding Netrin-1 and Slit-1/2. Explant assays revealed that the mutant RGCs exhibited disturbed Netrin-1-dependent axon outgrowth and Slit-2-dependent repulsion. CONCLUSIONS. The present study demonstrated that RGC axon projection toward the optic nerve head requires the expression of HS in the neural retina, suggesting that HS in the retina functions as an essential modulator of Netrin-1 and Slit-mediated intraretinal RGC axon guidance.
Figures






References
-
- Harada T, Harada C, Parada LF. Molecular regulation of visual system development: more than meets the eye. Genes Dev. 2007;21:367–378 - PubMed
-
- Inatani M. Molecular mechanisms of optic axon guidance. Naturwissenschaften. 2005;92:549–561 - PubMed
-
- Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–589 - PubMed
-
- Birgbauer E, Cowan CA, Sretavan DW, Henkemeyer M. Kinase independent function of EphB receptors in retinal axon pathfinding to the optic disc from dorsal but not ventral retina. Development. 2000;127:1231–1241 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

