Role of the Fas/FasL system in a model of RSV infection in mechanically ventilated mice
- PMID: 21743025
- PMCID: PMC3191752
- DOI: 10.1152/ajplung.00368.2010
Role of the Fas/FasL system in a model of RSV infection in mechanically ventilated mice
Abstract
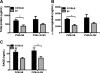
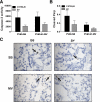
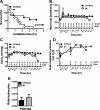
Infection with respiratory syncytial virus (RSV) in children can progress to respiratory distress and acute lung injury necessitating mechanical ventilation (MV). MV enhances apoptosis and inflammation in mice infected with pneumonia virus of mice (PVM), a mouse pneumovirus that has been used as a model for severe RSV infection in mice. We hypothesized that the Fas/Fas ligand (FasL) system, a dual proapoptotic/proinflammatory system involved in other forms of lung injury, is required for enhanced lung injury in mechanically ventilated mice infected with PVM. C57BL/6 mice and Fas-deficient ("lpr") mice were inoculated intratracheally with PVM. Seven or eight days after PVM inoculation, the mice were subjected to 4 h of MV (tidal volume 10 ml/kg, fraction of inspired O(2) = 0.21, and positive end-expiratory pressure = 3 cm H(2)O). Seven days after PVM inoculation, exposure to MV resulted in less severe injury in lpr mice than in C57BL/6 mice, as evidenced by decreased numbers of polymorphonuclear neutrophils in the bronchoalveolar lavage (BAL), and lower concentrations of the proinflammatory chemokines KC, macrophage inflammatory protein (MIP)-1α, and MIP-2 in the lungs. However, when PVM infection was allowed to progress one additional day, all of the lpr mice (7/7) died unexpectedly between 0.5 and 3.5 h after the onset of ventilation compared with three of the seven ventilated C57BL/6 mice. Parameters of lung injury were similar in nonventilated mice, as was the viral content in the lungs and other organs. Thus, the Fas/FasL system was partly required for the lung inflammatory response in ventilated mice infected with PVM, but attenuation of lung inflammation did not prevent subsequent mortality.
Figures
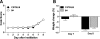


Similar articles
-
The Pneumonia Virus of Mice (PVM) model of acute respiratory infection.Viruses. 2012 Dec;4(12):3494-510. doi: 10.3390/v4123494. Viruses. 2012. PMID: 23342367 Free PMC article. Review.
-
Mechanical ventilation enhances lung inflammation and caspase activity in a model of mouse pneumovirus infection.Am J Physiol Lung Cell Mol Physiol. 2009 Jan;296(1):L46-56. doi: 10.1152/ajplung.00467.2007. Epub 2008 Nov 7. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 18996903 Free PMC article.
-
Fas-deficient mice have impaired alveolar neutrophil recruitment and decreased expression of anti-KC autoantibody:KC complexes in a model of acute lung injury.Respir Res. 2012 Oct 9;13(1):91. doi: 10.1186/1465-9921-13-91. Respir Res. 2012. PMID: 23043753 Free PMC article.
-
MIP-1alpha is produced but it does not control pulmonary inflammation in response to respiratory syncytial virus infection in mice.Cell Immunol. 2000 Nov 25;206(1):1-6. doi: 10.1006/cimm.2000.1730. Cell Immunol. 2000. PMID: 11161432
-
The pneumonia virus of mice infection model for severe respiratory syncytial virus infection: identifying novel targets for therapeutic intervention.Pharmacol Ther. 2005 Jan;105(1):1-6. doi: 10.1016/j.pharmthera.2004.09.001. Pharmacol Ther. 2005. PMID: 15626452 Review.
Cited by
-
The bioactivity of soluble Fas ligand is modulated by key amino acids of its stalk region.PLoS One. 2021 Jun 17;16(6):e0253260. doi: 10.1371/journal.pone.0253260. eCollection 2021. PLoS One. 2021. PMID: 34138914 Free PMC article.
-
Apoptotic cell death in disease-Current understanding of the NCCD 2023.Cell Death Differ. 2023 May;30(5):1097-1154. doi: 10.1038/s41418-023-01153-w. Epub 2023 Apr 26. Cell Death Differ. 2023. PMID: 37100955 Free PMC article. Review.
-
Respiratory syncytial virus infections enhance cigarette smoke induced COPD in mice.PLoS One. 2014 Feb 28;9(2):e90567. doi: 10.1371/journal.pone.0090567. eCollection 2014. PLoS One. 2014. PMID: 24587397 Free PMC article.
-
The Pneumonia Virus of Mice (PVM) model of acute respiratory infection.Viruses. 2012 Dec;4(12):3494-510. doi: 10.3390/v4123494. Viruses. 2012. PMID: 23342367 Free PMC article. Review.
-
The effect of TIP on pneumovirus-induced pulmonary edema in mice.PLoS One. 2014 Jul 21;9(7):e102749. doi: 10.1371/journal.pone.0102749. eCollection 2014. PLoS One. 2014. PMID: 25047452 Free PMC article.
References
-
- Altemeier WA, Matute-Bello G, Gharib SA, Glenny RW, Martin TR, Liles WC. Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol 175: 3369–3376, 2005 - PubMed
-
- Anh DB, Faisca P, Desmecht DJ. Differential resistance/susceptibility patterns to pneumovirus infection among inbred mouse strains. Am J Physiol Lung Cell Mol Physiol 291: L426–L435, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

