Flexibility in the patterning and control of axial locomotor networks in lamprey
- PMID: 21743089
- PMCID: PMC3223480
- DOI: 10.1093/icb/icr077
Flexibility in the patterning and control of axial locomotor networks in lamprey
Abstract
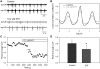
In lower vertebrates, locomotor burst generators for axial muscles generally produce unitary bursts that alternate between the two sides of the body. In lamprey, a lower vertebrate, locomotor activity in the axial ventral roots of the isolated spinal cord can exhibit flexibility in the timings of bursts to dorsally-located myotomal muscle fibers versus ventrally-located myotomal muscle fibers. These episodes of decreased synchrony can occur spontaneously, especially in the rostral spinal cord where the propagating body waves of swimming originate. Application of serotonin, an endogenous spinal neurotransmitter known to presynaptically inhibit excitatory synapses in lamprey, can promote decreased synchrony of dorsal-ventral bursting. These observations suggest the possible existence of dorsal and ventral locomotor networks with modifiable coupling strength between them. Intracellular recordings of motoneurons during locomotor activity provide some support for this model. Pairs of motoneurons innervating myotomal muscle fibers of similar ipsilateral dorsoventral location tend to have higher correlations of fast synaptic activity during fictive locomotion than do pairs of motoneurons innervating myotomes of different ipsilateral dorsoventral locations, suggesting their control by different populations of premotor interneurons. Further, these different motoneuron pools receive different patterns of excitatory and inhibitory inputs from individual reticulospinal neurons, conveyed in part by different sets of premotor interneurons. Perhaps, then, the locomotor network of the lamprey is not simply a unitary burst generator on each side of the spinal cord that activates all ipsilateral body muscles simultaneously. Instead, the burst generator on each side may comprise at least two coupled burst generators, one controlling motoneurons innervating dorsal body muscles and one controlling motoneurons innervating ventral body muscles. The coupling strength between these two ipsilateral burst generators may be modifiable and weakening when greater swimming maneuverability is required. Variable coupling of intrasegmental burst generators in the lamprey may be a precursor to the variable coupling of burst generators observed in the control of locomotion in the joints of limbed vertebrates.
Figures
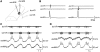


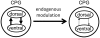
References
-
- Aoki F, Wannier T, Grillner S. Slow dorsal-ventral rhythm generator in the lamprey spinal cord. J Neurophysiol. 2001;85:211–8. - PubMed
-
- Brodin L, Buchanan JT, Hökfelt T, Grillner S, Verhofstad AAJ. A spinal projection of 5-hydroxytryptamine neurons in the lamprey brainstem; evidence from combined retrograde tracing and immunohistochemistry. Neurosci Lett. 1986;67:53–7. - PubMed
-
- Buchanan JT. Identification of interneurons with contralateral, caudal axons in the lamprey spinal cord: synaptic interactions and morphology. J Neurophysiol. 1982;47:961–75. - PubMed
-
- Buchanan JT. Neural network simulations of coupled locomotor oscillators in the lamprey spinal cord. Biol Cybern. 1992;66:367–74. - PubMed
-
- Buchanan JT. Electrophysiological properties of identified classes of lamprey spinal neurons. J Neurophysiol. 1993;70:2313–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

