Inhibitory effect of receptor for advanced glycation end products (RAGE) on the TGF-β-induced alveolar epithelial to mesenchymal transition
- PMID: 21743278
- PMCID: PMC3203242
- DOI: 10.3858/emm.2011.43.9.059
Inhibitory effect of receptor for advanced glycation end products (RAGE) on the TGF-β-induced alveolar epithelial to mesenchymal transition
Abstract
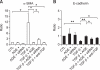
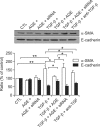
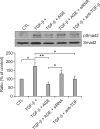
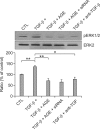
Idiopathic pulmonary fibrosis (IPF) is a lethal parenchymal lung disease characterized by myofibroblast proliferation. Alveolar epithelial cells (AECs) are thought to produce myofibroblasts through the epithelial to mesenchymal transition (EMT). Receptor for advanced glycation end products (RAGE) is a member of the immunoglobulin superfamily of cell surface receptors whose activation is associated with renal fibrosis during diabetes and liver fibrosis. RAGE is expressed at low basal levels in most adult tissues except the lung. In this study, we evaluated the interaction of ligand advanced glycation end products (AGE) with RAGE during the epithelial to myofibroblast transition in rat AECs. Our results indicate that AGE inhibited the TGF-β-dependent alveolar EMT by increasing Smad7 expression, and that the effect was abolished by RAGE siRNA treatment. Thus, the induction of Smad7 by the AGE-RAGE interaction limits the development of pulmonary fibrosis by inhibiting TGF-β-dependent signaling in AECs.
Figures




References
-
- Bargagli E, Penza F, Bianchi N, Olivieri C, Bennett D, Prasse A, Rottoli P. Controversial role of RAGE in the pathogenesis of idiopathic pulmonary fibrosis. Respir Physiol Neurobiol. 2009;165:119–120. author reply 121-2. - PubMed
-
- Bohlender JM, Franke S, Stein G, Wolf G. Advanced glycation end products and the kidney. Am J Physiol Renal Physiol. 2005;289:F645–F659. - PubMed
-
- Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. - PubMed
-
- Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, Nawroth PP. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198:1507–1515. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
