Synthesis and spectroscopic characterization of two tetrasubstituted cationic porphyrin derivatives
- PMID: 21743388
- PMCID: PMC6264253
- DOI: 10.3390/molecules16075807
Synthesis and spectroscopic characterization of two tetrasubstituted cationic porphyrin derivatives
Abstract
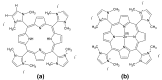
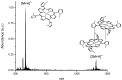
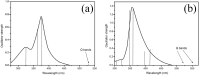
An imidazolium tetrasubstituted cationic porphyrin derivative (the free base and its Zn(II) complex) with five-membered heterocyclic groups in the meso-positions were synthesized using microwave irradiation, and the compounds obtained characterized by (1)H-NMR and mass spectrometry. We observed that under microwave irradiation the yield is similar to when the synthesis is performed under conventional heating, however, the time required to prepare the porphyrins decreases enormously. In order to investigate the electronic state of these compounds, we employed UV-Vis and fluorescence spectroscopy combined with quantum chemical calculations. The results reveal the presence, in both compounds, of a large number of electronic states involving the association between the Soret and a blue-shifted band. The Soret band in both compounds also shows a considerable solvent dependence. As for emission, these compounds present low quantum yield at room temperature and no solvent influence on the fluorescence spectra was observed.
Figures



Similar articles
-
The synthesis and characterisation of a free-base porphyrin-perylene dyad that exhibits electronic coupling in both the ground and excited states.Chemistry. 2009;15(1):248-53. doi: 10.1002/chem.200801779. Chemistry. 2009. PMID: 19021182
-
Ethyne Functionalized Meso-Phenothiazinyl-Phenyl-Porphyrins: Synthesis and Optical Properties of Free Base Versus Protonated Species.Molecules. 2020 Oct 4;25(19):4546. doi: 10.3390/molecules25194546. Molecules. 2020. PMID: 33020414 Free PMC article.
-
Synthesis and characterization of novel porphyrin-cinnamic acid conjugates.Spectrochim Acta A Mol Biomol Spectrosc. 2019 Dec 5;223:117314. doi: 10.1016/j.saa.2019.117314. Epub 2019 Jun 24. Spectrochim Acta A Mol Biomol Spectrosc. 2019. PMID: 31280126
-
Synthesis and characterization of 5,10,15,20-tetra[3-(3-trifluoromethyl)phenoxy] porphyrin.Molecules. 2007 Jul 11;12(7):1389-98. doi: 10.3390/12071389. Molecules. 2007. PMID: 17909494 Free PMC article.
-
Porphyrins with exocyclic rings. 14. Synthesis of tetraacenaphthoporphyrins, a new family of highly conjugated porphyrins with record-breaking long-wavelength electronic absorptions.J Org Chem. 2000 Mar 10;65(5):1530-9. doi: 10.1021/jo991730w. J Org Chem. 2000. PMID: 10814118
Cited by
-
Microwave synthesis under solvent-free conditions and spectral studies of some mesoporphyrinic complexes.Molecules. 2012 May 10;17(5):5592-603. doi: 10.3390/molecules17055592. Molecules. 2012. PMID: 22576229 Free PMC article.
-
Photophysical and Bactericidal Properties of Pyridinium and Imidazolium Porphyrins for Photodynamic Antimicrobial Chemotherapy.Molecules. 2021 Feb 20;26(4):1122. doi: 10.3390/molecules26041122. Molecules. 2021. PMID: 33672630 Free PMC article.
References
-
- Mathai S., Smith T.A., Ghiggino K.P. Singlet oxygen quantum yields of potential porphyrin-based photosensitisers for photodynamic therapy. Photochem. Photobiol. Sci. 2007;6:995–1002. - PubMed
-
- McDonald A.R., Franssen N., van Klink G.P.M., van Koten G. ‘Click’ silica immobilisation of metallo-porphyrin complexes and their application in epoxidation catalysis. J. Organomet. Chem. 2009;694:2153–2162. doi: 10.1016/j.jorganchem.2009.02.020. - DOI
-
- Yang S.I., Seth J., Strachan J.P., Gentemann S., Kim D., Holten D., Lindsey J.S., Bocian D.F. Ground and excited state electronic properties of halogenated tetraarylporphyrins. Tuning the building blocks for porphyrin-based photonic devices. J. Porph. Phthaloc. 1999;3:117–147. doi: 10.1002/(SICI)1099-1409(199902)3:2<117::AID-JPP110>3.0.CO;2-X. - DOI
-
- Campbell W.M., Jolley K.W., Wagner P., Wagner K., Walsh P.J., Gordon K.C., Schmidt-Mende L., Nazeeruddin M.K., Wang Q., Grätzel M., Officer D.L. Highly Efficient Porphyrin Sensitizers for Dye-Sensitized Solar Cells. J. Phys. Chem. C. 2007;111:11760–11762.
-
- Pavinatto F.J., Gameiro A.F., Jr., Hidalgo A.A., Dinelli L.R., Romualdo L.L., Batista A.A., Barbosa Neto N.M., Ferreira M., Oliveira O.N., Jr. Langmuir and Langmuir–Blodgett (LB) films of tetrapyridyl metalloporphyrins. Appl. Surf. Sci. 2008;254:5946–5952.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

