Control of Ubp3 ubiquitin protease activity by the Hog1 SAPK modulates transcription upon osmostress
- PMID: 21743437
- PMCID: PMC3160652
- DOI: 10.1038/emboj.2011.227
Control of Ubp3 ubiquitin protease activity by the Hog1 SAPK modulates transcription upon osmostress
Abstract
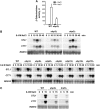
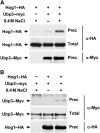
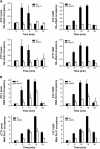
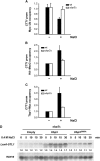
Protein ubiquitylation is a key process in the regulation of many cellular processes. The balance between the activity of ubiquitin ligases and that of proteases controls the level of ubiquitylation. In response to extracellular stimuli, stress-activated protein kinases (SAPK) modulate gene expression to maximize cell survival. In yeast, the Hog1 SAPK has a key role in reprogramming the gene expression pattern required for cell survival upon osmostress. Here, we show that the Ubp3 ubiquitin protease is a target for the Hog1 SAPK to modulate gene expression. ubp3 mutant cells are defective in expression of osmoresponsive genes. Hog1 interacts with and phosphorylates Ubp3 at serine 695, which is essential to determine the extent of transcriptional activation in response to osmostress. Furthermore, Ubp3 is recruited to osmoresponsive genes to modulate transcriptional initiation as well as elongation. Therefore, Ubp3 activity responds to external stimuli and is required for transcriptional activation upon osmostress.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Alepuz PM, Jovanovic A, Reiser V, Ammerer G (2001) Stress-induced map kinase Hog1 is part of transcription activation complexes. Mol Cell 7: 767–777 - PubMed
-
- Amerik AY, Hochstrasser M (2004) Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta 1695: 189–207 - PubMed
-
- Auty R, Steen H, Myers LC, Persinger J, Bartholomew B, Gygi SP, Buratowski S (2004) Purification of active TFIID from Saccharomyces cerevisiae. Extensive promoter contacts and co-activator function. J Biol Chem 279: 49973–49981 - PubMed
-
- Baker RT, Tobias JW, Varshavsky A (1992) Ubiquitin-specific proteases of Saccharomyces cerevisiae. Cloning of UBP2 and UBP3, and functional analysis of the UBP gene family. J Biol Chem 267: 23364–23375 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

