Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors
- PMID: 21743470
- PMCID: PMC3175737
- DOI: 10.1038/nn.2862
Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors
Abstract
Dopamine (DA) D2 receptors expressed in DA neurons (D2 autoreceptors) exert a negative feedback regulation that reduces DA neuron firing, DA synthesis and DA release. As D2 receptors are mostly expressed in postsynaptic neurons, pharmacological and genetic approaches have been unable to definitively address the in vivo contribution of D2 autoreceptors to DA-mediated behaviors. We found that midbrain DA neurons from mice deficient in D2 autoreceptors (Drd2(loxP/loxP); Dat(+/IRES-cre), referred to as autoDrd2KO mice) lacked DA-mediated somatodendritic synaptic responses and inhibition of DA release. AutoDrd2KO mice displayed elevated DA synthesis and release, hyperlocomotion and supersensitivity to the psychomotor effects of cocaine. The mice also exhibited increased place preference for cocaine and enhanced motivation for food reward. Our results highlight the importance of D2 autoreceptors in the regulation of DA neurotransmission and demonstrate that D2 autoreceptors are important for normal motor function, food-seeking behavior, and sensitivity to the locomotor and rewarding properties of cocaine.
Figures



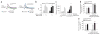

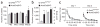
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

