Systems biology of vaccination for seasonal influenza in humans
- PMID: 21743478
- PMCID: PMC3140559
- DOI: 10.1038/ni.2067
Systems biology of vaccination for seasonal influenza in humans
Abstract
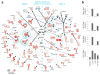
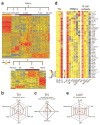
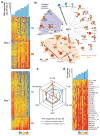
Here we have used a systems biology approach to study innate and adaptive responses to vaccination against influenza in humans during three consecutive influenza seasons. We studied healthy adults vaccinated with trivalent inactivated influenza vaccine (TIV) or live attenuated influenza vaccine (LAIV). TIV induced higher antibody titers and more plasmablasts than LAIV did. In subjects vaccinated with TIV, early molecular signatures correlated with and could be used to accurately predict later antibody titers in two independent trials. Notably, expression of the kinase CaMKIV at day 3 was inversely correlated with later antibody titers. Vaccination of CaMKIV-deficient mice with TIV induced enhanced antigen-specific antibody titers, which demonstrated an unappreciated role for CaMKIV in the regulation of antibody responses. Thus, systems approaches can be used to predict immunogenicity and provide new mechanistic insights about vaccines.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
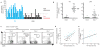
Comment in
-
Solving vaccine mysteries: a systems biology perspective.Nat Immunol. 2011 Jul 19;12(8):729-31. doi: 10.1038/ni.2078. Nat Immunol. 2011. PMID: 21772284 No abstract available.
References
-
- Fiore AE, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. - PubMed
-
- Zeman AM, et al. Humoral and cellular immune responses in children given annual immunization with trivalent inactivated influenza vaccine. Pediatr Infect Dis J. 2007;26:107–115. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- U19AI090023/AI/NIAID NIH HHS/United States
- R01DK057665/DK/NIDDK NIH HHS/United States
- U54AI057157/AI/NIAID NIH HHS/United States
- R37 DK057665/DK/NIDDK NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- R01 GM033976/GM/NIGMS NIH HHS/United States
- R37AI48638/AI/NIAID NIH HHS/United States
- UL1 RR025008/RR/NCRR NIH HHS/United States
- N01 AI030048/AI/NIAID NIH HHS/United States
- AI057266/AI/NIAID NIH HHS/United States
- R37 AI048638/AI/NIAID NIH HHS/United States
- HHSN266200700006C/AI/NIAID NIH HHS/United States
- R38 AI140299/AI/NIAID NIH HHS/United States
- U19 AI090023/AI/NIAID NIH HHS/United States
- R01 DK074701/DK/NIDDK NIH HHS/United States
- U19 AI057266/AI/NIAID NIH HHS/United States
- N01 AI050025/AI/NIAID NIH HHS/United States
- R01 DK057665/DK/NIDDK NIH HHS/United States
- U19AI057266/AI/NIAID NIH HHS/United States
- AI30048/AI/NIAID NIH HHS/United States
- DK074701/DK/NIDDK NIH HHS/United States
- N01 AI50025/AI/NIAID NIH HHS/United States
- U54 AI057157/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

