Cyclic AMP-guanine exchange factor activation inhibits JNK-dependent lipopolysaccharide-induced apoptosis in rat hepatocytes
- PMID: 21743791
- PMCID: PMC3131672
- DOI: 10.2147/HMER.S7673
Cyclic AMP-guanine exchange factor activation inhibits JNK-dependent lipopolysaccharide-induced apoptosis in rat hepatocytes
Abstract
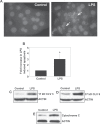
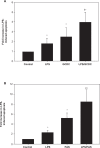
Lipopolysaccharide (LPS) is known to damage hepatocytes by cytokines released from activated Kupffer cells, but the ancillary role of LPS as a direct hepatotoxin is less well characterized. The aim of this study was to determine the direct effect of LPS on hepatocyte viability and the underlying signaling mechanism. Rat hepatocyte cultures treated overnight with LPS (500 ng/mL) induced apoptosis as monitored morphologically (Hoechst 33258) and biochemically (cleavage of caspase 3 and 9 and the appearance of cytochrome C in the cytoplasm). LPS-induced apoptosis was additive to that induced by glycochenodeoxycholate or Fas ligand, was associated with activation of c-Jun N-terminal kinase B (JNK) and p38 mitogen-activated protein kinases (MAPK), and inhibition of protein kinase (AKT). Inhibition of JNK by SP600125, but not of p38 MAPK by SB203580 attenuated LPS-induced apoptosis, indicating JNK dependency. CPT-2-Me-cAMP, an activator of cAMP-GEF, decreased apoptosis due to LPS alone or in combination with glycochenodeoxycholate or Fas ligand. CPT-2-Me-cAMP also prevented LPS-induced activation of JNK and inhibition of AKT. Taken together, these results suggest that LPS can induce hepatocyte apoptosis directly in vitro in a JNK-dependent manner and activation of cAMP-GEF protects against the LPS-induced apoptosis most likely by reversing the effect of LPS on JNK and AKT.
Figures



Similar articles
-
cAMP-guanine exchange factor protection from bile acid-induced hepatocyte apoptosis involves glycogen synthase kinase regulation of c-Jun NH2-terminal kinase.Am J Physiol Gastrointest Liver Physiol. 2011 Aug;301(2):G385-400. doi: 10.1152/ajpgi.00430.2010. Epub 2011 May 5. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21546580 Free PMC article.
-
Activation of cAMP-guanine exchange factor confers PKA-independent protection from hepatocyte apoptosis.Am J Physiol Gastrointest Liver Physiol. 2004 Aug;287(2):G334-43. doi: 10.1152/ajpgi.00517.2003. Epub 2004 Mar 25. Am J Physiol Gastrointest Liver Physiol. 2004. PMID: 15044179
-
Activation of focal adhesion kinase and JNK contributes to the extracellular matrix and cAMP-GEF mediated survival from bile acid induced apoptosis in rat hepatocytes.J Hepatol. 2008 Aug;49(2):251-61. doi: 10.1016/j.jhep.2008.04.015. Epub 2008 May 22. J Hepatol. 2008. PMID: 18550202 Free PMC article.
-
cAMP-GEF cytoprotection by Src tyrosine kinase activation of phosphoinositide-3-kinase p110 beta/alpha in rat hepatocytes.Am J Physiol Gastrointest Liver Physiol. 2009 Apr;296(4):G764-74. doi: 10.1152/ajpgi.90622.2008. Epub 2009 Feb 5. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19196950 Free PMC article.
-
Cytochrome P4502E1 sensitizes to tumor necrosis factor alpha-induced liver injury through activation of mitogen-activated protein kinases in mice.Hepatology. 2008 Mar;47(3):1005-17. doi: 10.1002/hep.22087. Hepatology. 2008. PMID: 18095305
Cited by
-
Hepatic expression of lipopolysaccharide-binding protein (Lbp) is induced by the gut microbiota through Myd88 and impairs glucose tolerance in mice independent of obesity.Mol Metab. 2020 Jul;37:100997. doi: 10.1016/j.molmet.2020.100997. Epub 2020 Apr 16. Mol Metab. 2020. PMID: 32305515 Free PMC article.
-
cAMP-guanine exchange factor protection from bile acid-induced hepatocyte apoptosis involves glycogen synthase kinase regulation of c-Jun NH2-terminal kinase.Am J Physiol Gastrointest Liver Physiol. 2011 Aug;301(2):G385-400. doi: 10.1152/ajpgi.00430.2010. Epub 2011 May 5. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21546580 Free PMC article.
-
8-Chloroadenosine 3',5'-monophosphate induces cell cycle arrest and apoptosis in multiple myeloma cells through multiple mechanisms.Oncol Lett. 2012 Dec;4(6):1384-1388. doi: 10.3892/ol.2012.905. Epub 2012 Sep 11. Oncol Lett. 2012. PMID: 23226809 Free PMC article.
-
The effects of ursodeoxycholic acid on sepsis-induced cholestasis management in an animal model.J Taibah Univ Med Sci. 2020 Jun 27;15(4):312-320. doi: 10.1016/j.jtumed.2020.04.007. eCollection 2020 Aug. J Taibah Univ Med Sci. 2020. PMID: 32982635 Free PMC article.
-
Quantitative proteomic analysis of hepatocyte-secreted extracellular vesicles reveals candidate markers for liver toxicity.J Proteomics. 2014 May 30;103:227-40. doi: 10.1016/j.jprot.2014.04.008. Epub 2014 Apr 18. J Proteomics. 2014. PMID: 24747303 Free PMC article.
References
-
- Jirillo E, Caccavo D, Magrone T, et al. The role of the liver in the response to LPS; experimental and clinical findings. J Endotoxin Res. 2002;8:319–327. - PubMed
-
- Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology. 2006;130:1886–1900. - PubMed
-
- Szabo G, Dolganiuc A, Mandrekar P. Pattern recognition receptors: A contemporary view of liver disease. Hepatology. 2006;44:287–298. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

