Cyclic AMP: a selective modulator of NF-κB action
- PMID: 21744067
- PMCID: PMC11114830
- DOI: 10.1007/s00018-011-0757-8
Cyclic AMP: a selective modulator of NF-κB action
Abstract
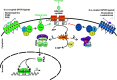
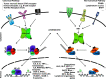
It has been known for several decades that cyclic AMP (cAMP), a prototypical second messenger, transducing the action of a variety of G-protein-coupled receptor ligands, has potent immunosuppressive and anti-inflammatory actions. These actions have been attributed in part to the ability of cAMP-induced signals to interfere with the function of the proinflammatory transcription factor Nuclear Factor-kappaB (NF-κB). NF-κB plays a crucial role in switching on the gene expression of a plethora of inflammatory and immune mediators, and as such is one of the master regulators of the immune response and a key target for anti-inflammatory drug design. A number of fundamental molecular mechanisms, contributing to the overall inhibitory actions of cAMP on NF-κB function, are well established. Paradoxically, recent reports indicate that cAMP, via its main effector, the protein kinase A (PKA), also promotes NF-κB activity. Indeed, cAMP actions appear to be highly cell type- and context-dependent. Importantly, several novel players in the cAMP/NF-κB connection, which selectively direct cAMP action, have been recently identified. These findings not only open up exciting new research avenues but also reveal novel opportunities for the design of more selective, NF-κB-targeting, anti-inflammatory drugs.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

