Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis
- PMID: 21744074
- PMCID: PMC3188710
- DOI: 10.1007/s00125-011-2232-3
Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis
Abstract
Aims/hypothesis: The innate immune cells, invariant natural killer T cells (iNKT cells), are implicated in the pathogenesis of psoriasis, an inflammatory condition associated with obesity and other metabolic diseases, such as diabetes and dyslipidaemia. We observed an improvement in psoriasis severity in a patient within days of starting treatment with an incretin-mimetic, glucagon-like peptide-1 (GLP-1) receptor agonist. This was independent of change in glycaemic control. We proposed that this unexpected clinical outcome resulted from a direct effect of GLP-1 on iNKT cells.
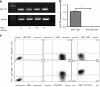
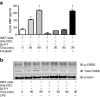
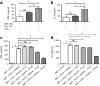
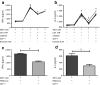
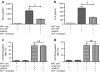
Methods: We measured circulating and psoriatic plaque iNKT cell numbers in two patients with type 2 diabetes and psoriasis before and after commencing GLP-1 analogue therapy. In addition, we investigated the in vitro effects of GLP-1 on iNKT cells and looked for a functional GLP-1 receptor on these cells.
Results: The Psoriasis Area and Severity Index improved in both patients following 6 weeks of GLP-1 analogue therapy. This was associated with an alteration in iNKT cell number, with an increased number in the circulation and a decreased number in psoriatic plaques. The GLP-1 receptor was expressed on iNKT cells, and GLP-1 induced a dose-dependent inhibition of iNKT cell cytokine secretion, but not cytolytic degranulation in vitro.
Conclusions/interpretation: The clinical effect observed and the direct interaction between GLP-1 and the immune system raise the possibility of therapeutic applications for GLP-1 in inflammatory conditions such as psoriasis.
Figures
Comment in
-
Glucagon-like peptide-1 (GLP-1) receptor agonists, obesity and psoriasis: diabetes meets dermatology.Diabetologia. 2011 Nov;54(11):2741-4. doi: 10.1007/s00125-011-2297-z. Epub 2011 Sep 3. Diabetologia. 2011. PMID: 21892687
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

