Total chemical synthesis of biologically active vascular endothelial growth factor
- PMID: 21744452
- PMCID: PMC3478761
- DOI: 10.1002/anie.201103237
Total chemical synthesis of biologically active vascular endothelial growth factor
Abstract
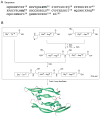
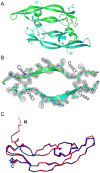
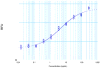
Efficient access: the 204-residue covalent-dimer vascular endothelial growth factor with full mitogenic activity was prepared from three unprotected peptide segments by one-pot native chemical ligations. The covalent structure of the synthetic VEGF was confirmed by precise mass measurement, and the three-dimensional structure of the synthetic protein was determined by high-resolution X-ray crystallography.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

Similar articles
-
Chemical synthesis and X-ray structure of a heterochiral {D-protein antagonist plus vascular endothelial growth factor} protein complex by racemic crystallography.Proc Natl Acad Sci U S A. 2012 Sep 11;109(37):14779-84. doi: 10.1073/pnas.1210483109. Epub 2012 Aug 27. Proc Natl Acad Sci U S A. 2012. PMID: 22927390 Free PMC article.
-
Solution structure of a phage-derived peptide antagonist in complex with vascular endothelial growth factor.J Mol Biol. 2002 Feb 22;316(3):769-87. doi: 10.1006/jmbi.2001.5370. J Mol Biol. 2002. PMID: 11866530
-
Total chemical synthesis and X-ray structure of kaliotoxin by racemic protein crystallography.Chem Commun (Camb). 2010 Nov 21;46(43):8174-6. doi: 10.1039/c0cc03148h. Epub 2010 Sep 28. Chem Commun (Camb). 2010. PMID: 20877851
-
Through the looking glass--a new world of proteins enabled by chemical synthesis.J Pept Sci. 2012 Jul;18(7):428-36. doi: 10.1002/psc.2421. Epub 2012 Jun 4. J Pept Sci. 2012. PMID: 22674813 Review.
-
Synthesis of native proteins by chemical ligation.Annu Rev Biochem. 2000;69:923-60. doi: 10.1146/annurev.biochem.69.1.923. Annu Rev Biochem. 2000. PMID: 10966479 Review.
Cited by
-
Novel protein science enabled by total chemical synthesis.Protein Sci. 2019 Feb;28(2):313-328. doi: 10.1002/pro.3533. Epub 2018 Dec 18. Protein Sci. 2019. PMID: 30345579 Free PMC article. Review.
-
The ER stress response mediator ERO1 triggers cancer metastasis by favoring the angiogenic switch in hypoxic conditions.Oncogene. 2021 Mar;40(9):1721-1736. doi: 10.1038/s41388-021-01659-y. Epub 2021 Feb 2. Oncogene. 2021. PMID: 33531624 Free PMC article.
-
Design, Synthesis, and Biological Evaluations of a Novel Resveratrol-Type Analogue Against VEGF.Molecules. 2025 May 27;30(11):2345. doi: 10.3390/molecules30112345. Molecules. 2025. PMID: 40509232 Free PMC article.
-
De novo design of D-peptide ligands: Application to influenza virus hemagglutinin.Proc Natl Acad Sci U S A. 2025 Jul;122(26):e2426554122. doi: 10.1073/pnas.2426554122. Epub 2025 Jun 27. Proc Natl Acad Sci U S A. 2025. PMID: 40577121
-
Efficient Chemical Protein Synthesis using Fmoc-Masked N-Terminal Cysteine in Peptide Thioester Segments.Angew Chem Int Ed Engl. 2020 Aug 24;59(35):14796-14801. doi: 10.1002/anie.202000491. Epub 2020 May 26. Angew Chem Int Ed Engl. 2020. PMID: 32333711 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

