The influence of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on osteoclastogenesis
- PMID: 21745106
- PMCID: PMC3148120
- DOI: 10.1089/thy.2010.0457
The influence of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on osteoclastogenesis
Abstract
Background: We have shown that thyroid-stimulating hormone (TSH) has a direct inhibitory effect on osteoclastic bone resorption and that TSH receptor (TSHR) null mice display osteoporosis. To determine the stage of osteoclast development at which TSH may exert its effect, we examined the influence of TSH and agonist TSHR antibodies (TSHR-Ab) on osteoclast differentiation from murine embryonic stem (ES) cells to gain insight into bone remodeling in hyperthyroid Graves' disease.
Methods: Osteoclast differentiation was initiated in murine ES cell cultures through exposure to macrophage colony stimulation factor, receptor activator of nuclear factor кB ligand, vitamin D, and dexamethasone.
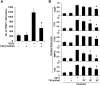
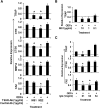
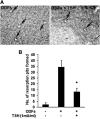
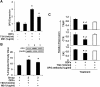
Results: Tartrate resistant acid phosphatase (TRAP)-positive osteoclasts formed in ~12 days. This coincided with the expected downregulation of known markers of self renewal and pluripotency (including Oct4, Sox2, and REX1). Both TSH and TSHR-Abs inhibited osteoclastogenesis as evidenced by decreased development of TRAP-positive cells (~40%-50% reduction, p = 0.0047), and by decreased expression, in a concentration-dependent manner, of osteoclast differentiation markers (including the calcitonin receptor, TRAP, cathepsin K, matrix metallo-proteinase-9, and carbonic anhydrase II). Similar data were obtained using serum immunoglobulin-Gs (IgGs) from patients with hyperthyroid Graves' disease and known TSHR-Abs. TSHR stimulators inhibited tumor necrosis factor-alpha mRNA and protein expression, but increased the expression of osteoprotegerin (OPG), an antiosteoclastogenic human soluble receptor activator of nuclear factor кB ligand receptor. Neutralizing antibody to OPG reversed the inhibitory effect of TSH on osteoclast differentiation evidencing that the TSH effect was at least in part mediated by increased OPG.
Conclusion: These data establish ES-derived osteoclastogenesis as an effective model system to study the regulation of osteoclast differentiation in early development. The results support the observations that TSH has a bone protective action by negatively regulating osteoclastogenesis. Further, our results implicate TSHR-Abs in offering skeletal protection in hyperthyroid Graves' disease, even in the face of high thyroid hormone and low TSH levels.
Figures




References
-
- Ross DS. Hyperthyroidism, thyroid hormone therapy, and bone. Thyroid. 1994;4:319–326. - PubMed
-
- Biondi B. Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76–131. - PubMed
-
- Zaidi M. Davies TF. Zallone A. Blair HC. Iqbal J. Moonga SS. Mechanick J. Sun L. Thyroid-stimulating hormone, thyroid hormones, and bone loss. Curr Osteoporos Rep. 2009;7:47–52. - PubMed
-
- Kim BJ. Lee SH. Bae SJ. Kim HK. Choe JW. Kim HY. Koh JM. Kim GS. The association between serum thyrotropin (TSH) levels and bone mineral density in healthy euthyroid men. Clin Endocrinol (Oxf) 2010;73:396–403. - PubMed
-
- Mazziotti G. Porcelli T. Patelli I. Vescovi PP. Giustina A. Serum TSH values and risk of vertebral fractures in euthyroid post-menopausal women with low bone mineral density. Bone. 2010;46:747–751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

