Tamsulosin shows a higher unbound drug fraction in human prostate than in plasma: a basis for uroselectivity?
- PMID: 21745239
- PMCID: PMC3162651
- DOI: 10.1111/j.1365-2125.2010.03870.x
Tamsulosin shows a higher unbound drug fraction in human prostate than in plasma: a basis for uroselectivity?
Abstract
What is already known about this subject: The efficacy-tolerability profile of tamsulosin in patients with benign prostatic hyperplasia (BPH) is assumed to be associated both with the α1-adrenoceptor selectivity profile of the drug and a small peak : trough ratio in the plasma pharmacokinetic (PK) profile. Tamsulosin is highly bound to plasma proteins, notably α1-acid glycoprotein (AGP). This protein is a high-affinity binding protein and AGP plasma concentration was found to influence the therapeutic (unbound) plasma concentrations for high-AGP-binding drugs.
What this study adds: The study actually assessed unbound tamsulosin concentrations in both blood plasma and prostate tissue and reported that the unbound tamsulosin concentrations after multiple dosing in men with BPH, were much higher in prostate than in blood plasma. The assumption is put forward that differential free drug concentrations in prostate and blood plasma may contribute to the relative ‘uroselectivity’ of tamsulosin.
Aim: The aim of this small patient study was to investigate tamsulosin concentrations in prostate and plasma samples in order to identify potential differences in the pharmacokinetics (PK) in plasma and prostate contributing to its pharmacodynamic activity profile in patients.
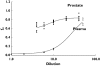
Methods: Forty-one patients with benign prostatic hyperplasia (BPH) scheduled for open prostatectomy were given tamsulosin 0.4 mg for 6-21 days in order to reach steady-state PK. Patients were randomized over four groups to allow collection of plasma and tissue samples at different time points after last dose administration. Samples were collected during surgery and assayed for tamsulosin HCl. The free fraction (f(u)) of tamsulosin was determined by ultracentrifugation of plasma and prostate tissue spiked with (14)C-tamsulosin.
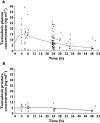
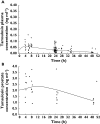
Results: C(max) in plasma at 4.4 h for total tamsulosin was 15.2 ng ml(-1) and AUC(0,24 h) was 282 ng ml(-1) h, while for prostate C(max) at 11.4 h post-dose was 5.4 ng ml(-1) and AUC(0,24 h) was 120 ng ml(-1) h. AUC(0,24 h) for total tamsulosin in prostate was 43% of the plasma AUC(0,24 h). f(u) was 0.4 % for plasma and 59.1% for prostate. Therefore calculated on unbound tamsulosin, a ratio of 63 resulted for prostate vs. plasma C(max) concentrations.
Conclusions: These data indicate that in patients with confirmed BPH the amount of tamsulosin freely available in the target tissue (prostate) is much higher than in plasma.
© 2011 Astellas Pharma Europe B.V. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures
References
-
- Chapple CR. Medical therapy and quality of life. Eur Urol. 1998;34(Suppl 1):10–7. - PubMed
-
- De Mey C. α1-blockers for BPH: are there differences? Eur Urol. 1999;36(Suppl 1):52–63. - PubMed
-
- Testa R, Sironi G, Colombo D, Greto L, Leonardi A. Rec 15/2739 a new α1-antagonist selective for the lower urinary tract: in vivo studies. Neurourol Urodyn. 1994;13:471–3.
-
- Martin DJ, LLuel P, Guillot E, Coste A, Jammes D, Angel I. Comparative alpha-1 adrenoceptor subtype selectivity and functional uroselectivity of alpha-1 adrenoceptor antagonists. J Pharmacol Exp Ther. 1997;282:228–35. - PubMed
-
- Blue DR, Jr, Grino P, Jung DT, Harbison MP, Ford ADPW. Evaluation of Ro 70-0004, a selective α1A-adrenoceptor antagonist, in men with benign prostatic hyperplasia (BPH) [abstract] Proc Fifth International Consultation BPH. 2000:550.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

