Myosin VI contributes to synaptic transmission and development at the Drosophila neuromuscular junction
- PMID: 21745401
- PMCID: PMC3146895
- DOI: 10.1186/1471-2202-12-65
Myosin VI contributes to synaptic transmission and development at the Drosophila neuromuscular junction
Abstract
Background: Myosin VI, encoded by jaguar (jar) in Drosophila melanogaster, is a unique member of the myosin superfamily of actin-based motor proteins. Myosin VI is the only myosin known to move towards the minus or pointed ends of actin filaments. Although Myosin VI has been implicated in numerous cellular processes as both an anchor and a transporter, little is known about the role of Myosin VI in the nervous system. We previously recovered jar in a screen for genes that modify neuromuscular junction (NMJ) development and here we report on the genetic analysis of Myosin VI in synaptic development and function using loss of function jar alleles.
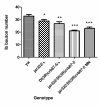
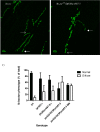
Results: Our experiments on Drosophila third instar larvae revealed decreased locomotor activity, a decrease in NMJ length, a reduction in synaptic bouton number, and altered synaptic vesicle localization in jar mutants. Furthermore, our studies of synaptic transmission revealed alterations in both basal synaptic transmission and short-term plasticity at the jar mutant neuromuscular synapse.
Conclusions: Altogether these findings indicate that Myosin VI is important for proper synaptic function and morphology. Myosin VI may be functioning as an anchor to tether vesicles to the bouton periphery and, thereby, participating in the regulation of synaptic vesicle mobilization during synaptic transmission.
Figures

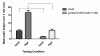





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

