HIV-1 Tat binds to SH3 domains: cellular and viral outcome of Tat/Grb2 interaction
- PMID: 21745501
- PMCID: PMC3527102
- DOI: 10.1016/j.bbamcr.2011.06.012
HIV-1 Tat binds to SH3 domains: cellular and viral outcome of Tat/Grb2 interaction
Abstract
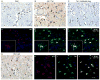
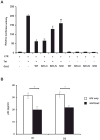
The Src-homology 3 (SH3) domain is one of the most frequent protein recognition modules (PRMs), being represented in signal transduction pathways and in several pathologies such as cancer and AIDS. Grb2 (growth factor receptor-bound protein 2) is an adaptor protein that contains two SH3 domains and is involved in receptor tyrosine kinase (RTK) signal transduction pathways. The HIV-1 transactivator factor Tat is required for viral replication and it has been shown to bind directly or indirectly to several host proteins, deregulating their functions. In this study, we show interaction between the cellular factor Grb2 and the HIV-1 trans-activating protein Tat. The binding is mediated by the proline-rich sequence of Tat and the SH3 domain of Grb2. As the adaptor protein Grb2 participates in a wide variety of signaling pathways, we characterized at least one of the possible downstream effects of the Tat/Grb2 interaction on the well-known IGF-1R/Raf/MAPK cascade. We show that the binding of Tat to Grb2 impairs activation of the Raf/MAPK pathway, while potentiating the PKA/Raf inhibitory pathway. The Tat/Grb2 interaction affects also viral function by inhibiting the Tat-mediated transactivation of HIV-1 LTR and viral replication in infected primary microglia.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

