A BAC transgenic Hes1-EGFP reporter reveals novel expression domains in mouse embryos
- PMID: 21745596
- PMCID: PMC3163761
- DOI: 10.1016/j.gep.2011.06.004
A BAC transgenic Hes1-EGFP reporter reveals novel expression domains in mouse embryos
Abstract
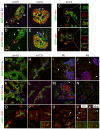
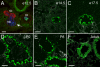
Expression of the basic helix-loop-helix factor Hairy and Enhancer of Split-1 (Hes1) is required for normal development of a number of tissues during embryonic development. Depending on context, Hes1 may act as a Notch signalling effector which promotes the undifferentiated and proliferative state of progenitor cells, but increasing evidence also points to Notch independent regulation of Hes1 expression. Here we use high resolution confocal scanning of EGFP in a novel BAC transgenic mouse reporter line, Tg(Hes1-EGFP)(1Hri), to analyse Hes1 expression from embryonic day 7.0 (e7.0). Our data recapitulates some previous observations on Hes1 expression and suggests new, hitherto unrecognised expression domains including expression in the definitive endoderm at early somite stages before gut tube closure and thus preceding organogenesis. This mouse line will be a valuable tool for studies addressing the role of Hes1 in a number of different research areas including organ specification, development and regeneration.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures






References
-
- Ahnfelt-Ronne J, Jorgensen MC, Hald J, Madsen OD, Serup P, Hecksher-Sorensen J. An improved method for three-dimensional reconstruction of protein expression patterns in intact mouse and chicken embryos and organs. J Histochem Cytochem. 2007;55:925–930. - PubMed
-
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. - PubMed
-
- Burke Z, Oliver G. Prox1 is an early specific marker for the developing liver and pancreas in the mammalian foregut endoderm. Mech Dev. 2002;118:147–155. - PubMed
-
- Chen L, Al-Awqati Q. Segmental expression of Notch and Hairy genes in nephrogenesis. Am J Physiol Renal Physiol. 2005;288:F939–952. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

