The immunoglobulin super family protein RIG-3 prevents synaptic potentiation and regulates Wnt signaling
- PMID: 21745641
- PMCID: PMC3134796
- DOI: 10.1016/j.neuron.2011.05.034
The immunoglobulin super family protein RIG-3 prevents synaptic potentiation and regulates Wnt signaling
Abstract
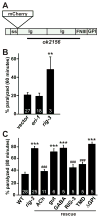
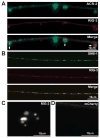
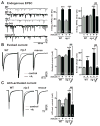
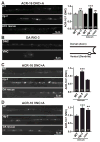
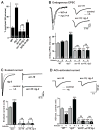
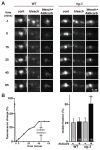
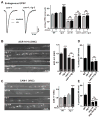
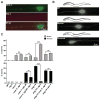
Cell surface Ig superfamily proteins (IgSF) have been implicated in several aspects of neuron development and function. Here, we describe the function of a Caenorhabditis elegans IgSF protein, RIG-3. Mutants lacking RIG-3 have an exaggerated paralytic response to a cholinesterase inhibitor, aldicarb. Although RIG-3 is expressed in motor neurons, heightened drug responsiveness was caused by an aldicarb-induced increase in muscle ACR-16 acetylcholine receptor (AChR) abundance, and a corresponding potentiation of postsynaptic responses at neuromuscular junctions. Mutants lacking RIG-3 also had defects in the anteroposterior polarity of the ALM mechanosensory neurons. The effects of RIG-3 on synaptic transmission and ALM polarity were both mediated by changes in Wnt signaling, and in particular by inhibiting CAM-1, a Ror-type receptor tyrosine kinase that binds Wnt ligands. These results identify RIG-3 as a regulator of Wnt signaling, and suggest that RIG-3 has an anti-plasticity function that prevents activity-induced changes in postsynaptic receptor fields.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
References
-
- Barrow AD, Trowsdale J. The extended human leukocyte receptor complex: diverse ways of modulating immune responses. Immunol Rev. 2008;224:98–123. - PubMed
-
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

