Cellular strategies of protein quality control
- PMID: 21746797
- PMCID: PMC3140689
- DOI: 10.1101/cshperspect.a004374
Cellular strategies of protein quality control
Abstract
Eukaryotic cells must contend with a continuous stream of misfolded proteins that compromise the cellular protein homeostasis balance and jeopardize cell viability. An elaborate network of molecular chaperones and protein degradation factors continually monitor and maintain the integrity of the proteome. Cellular protein quality control relies on three distinct yet interconnected strategies whereby misfolded proteins can either be refolded, degraded, or delivered to distinct quality control compartments that sequester potentially harmful misfolded species. Molecular chaperones play a critical role in determining the fate of misfolded proteins in the cell. Here, we discuss the spatial and temporal organization of cellular quality control strategies and their implications for human diseases linked to protein misfolding and aggregation.
Figures

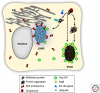
References
-
- Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T 2003. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science 299: 1751–1753 - PubMed
-
- Albanese V, Yam AY, Baughman J, Parnot C, Frydman J 2006. Systems analyses reveal two chaperone networks with distinct functions in eukaryotic cells. Cell 124: 75–88 - PubMed
-
- Alberti S, Demand J, Esser C, Emmerich N, Schild H, Hohfeld J 2002. Ubiquitylation of BAG-1 suggests a novel regulatory mechanism during the sorting of chaperone substrates to the proteasome. J Biol Chem 277: 45920–45927 - PubMed
-
- An S, Kumar R, Sheets ED, Benkovic SJ 2008. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science 320: 103–106 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
